Fig. 14.1
Five-year overall survival by actual procedure for all patients randomized by surgeons with a lower than average conversion rate (log rank test): p = 0.013 (Wilcoxon test) (From Jayne et al. [14])
Functional Outcome
Urinary and sexual dysfunction following rectal resection for cancer has historically been reported as being as high as 30–40%, especially after APR. The adoption of TME dissection and autonomic nerve sparing techniques have gone some way to reducing this, however there are still risks to urinary and sexual function.
Quah et al. showed a greater deterioration in sexual activity after laparoscopic compared to open laparoscopic surgery [18].
Jayne found similar outcomes in his analysis of the CLASICC trial data [19]. Of the 247 (71.2 %) who completed questionaries 98 had a laparoscopic rectal resection and 50 an open resection. The rate of severe sexual function change in males following open rectal resection was 23 %, in keeping with previous studies. Sexual function tended to be worse after laparoscopic rectal TME, especially for erectile dysfunction. Performance of a complete TME gave a sixfold increase in the chance of postoperative sexual dysfunction compared to a partial mesorectal dissection.
A recent systematic review, seeking to synthesize expert opinion in the literature found that while there had been growing acceptance of laparoscopic colonic surgery for some time, a laparoscopic approach to rectal cancer remains controversial (Fig. 14.2) [20]. There is no doubt that laparoscopic proctectomy is a complex procedure, however in expert hands, the current evidence would suggest that long term oncologic outcomes are similar to an open approach but more randomized, long term data is needed.
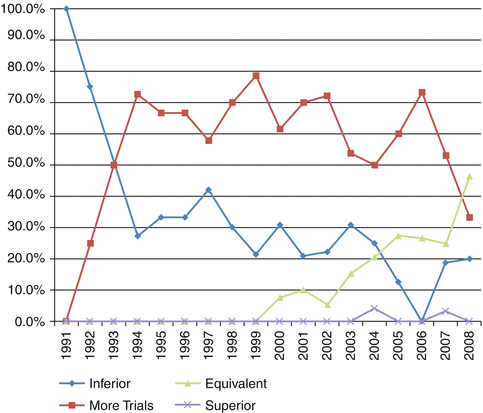

Fig. 14.2
Temporal summary of expert opinion in the literature pertaining to laparoscopic surgery for rectal cancer (From Martel et al. [20])
Robotic- Assisted Proctectomy
Robotic-assisted proctectomy, undertaken using the da VinciTM system (Intuitive Surgical, Sunnyvale, CA, USA) was first reported in 2003 [21]. It is apparent that there are a number of features of the robotic system that have the potential to reduce or eliminate some of the inherent problems associated with a laparoscopic approach.
The operating surgeon can be comfortably seated at the robotic console that presents a stable three-dimensional high-definition image (Fig. 14.3). Three operating and one camera arms are mounted on a patient-side cart with the instruments being inserted through ports similar to standard laparoscopic ports. The ports themselves are positioned so the ‘zero-point’ of the robotic instrument is centred in the patient’s abdominal wall. The movements of the arm and instrument are coordinated to keep this point stationary to minimise trauma to the abdominal wall. The surgeon is in full control of the laparoscopic camera and is not reliant on an assistant to maintain position or orientation. The robotic instruments are wristed and capable of precise dexterous movements controlled by the surgeon through an intuitive console system (Fig. 14.4). Instrument movement can be scaled and tremor eliminated. The console does not provide haptic feedback that can make discrimination of tissue consistency difficult and requires care when placing traction on delicate structures or when knot-tying. Movement is possible through 7° of freedom, 180° of articulation and 540° of rotation [22]. The robot itself has undergone a number of iterations, initial versions of the robot had one fewer operating arm and a more limited range of movement requiring adaption of docking techniques in order for the operating instruments to adequately reach all quadrants of the abdomen. At this point, the third generation, da Vinci Si HD is the current evolution.

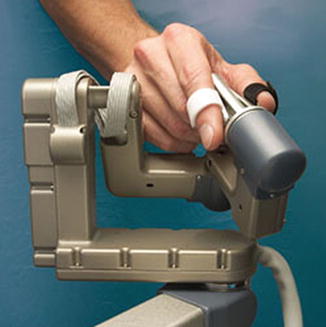

Fig. 14.3
The surgeon at the console and the patient side cart of the da Vinci Si HD Surgical System (http://www.intuitivesurgical.com) (From Freschi et al. [22])

Fig. 14.4
The daVinci master control interface used to remotely manipulate the wristed robotic instruments tip (From Freschi et al. [22])
Technique
Given that a proportion of the colonic mobilisation in proctectomy does not require the precise manipulative skills of the robot a number of ‘hybrid’ techniques have been developed where the initial component of the dissection is performed laparoscopically: usually the splenic flexure take-down, and then the cart is docked for IMA mobilisation and division and pelvic dissection.
Port placement is dependent of whether a hybrid or totally robotic technique is being undertaken. In either case the 12 mm camera port is usually placed just above and to the right of the umbilicus. It is important to allow adequate spacing of 8–10 cm between ports and an adequate distance from the pelvis to minimise arm clashing. Pneumoperitoneum should be established prior to placing the working ports to ensure satisfactory positioning.
Two 8 mm ports are then placed under direct vision on either side just lateral to the midclavicular line (MCL) on a line between the umbilicus and anterior superior iliac spine. For a single stage totally robotic procedure, a third 8 mm port is placed in the right upper quadrant (RUQ) in the MCL, and a fourth 8 mm port in the left upper quadrant (LUQ) just lateral to the MCL. A 5 mm assistant port is placed laterally in the right upper quadrant, and is used for added retraction, suction and irrigation [23, 24]. For a hybrid approach, the right subcostal port is omitted and the LUQ port can be brought caudally, providing an appropriate distance from the other ports is maintained (Fig. 14.5) [25].
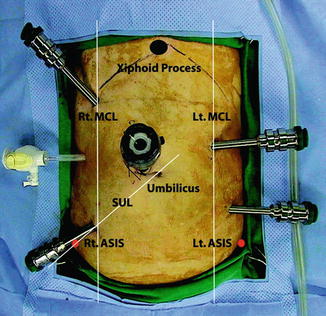

Fig. 14.5
The layout of the port placement for single-stage robotic low anterior resection. MCL midclavicular line, SUL spinoumbilical line, ASIS anterior superior iliac spine, Rt. right, Lt. left (From Choi et al. [23])
The patient is positioned in steep Trendelenburg position, left side up and the abdomen explored laparoscopically. The small bowel is retracted out of the pelvis, and at this point the colon can be mobilised laparoscopically and the splenic flexure taken down. One disadvantage of the robot is that, once docked, the patient position cannot be changed without undocking and then redocking the robotic cart, thereby negating the helpful effect of gravity when mobilising the splenic flexure.
The robot can then be docked with the patient cart approaching and docking over the left leg (Fig. 14.6). If the inferior mesenteric vessels are to be dissected, the robotic arms are first docked with robot arm 1 in the right lower quadrant for a monopolar shears, robot arm 2 in the LUQ port for a Cadiere grasper, and robot arm 3 in the RUQ for a bipolar grasper. The mesocolon over the IMA is retracted upwards and the peritoneum opened at its base. The IMA is dissected, care being taken to protect the periaortic hypogastric nerve plexus. The IMA can be divided near its base with laparoscopic or robotic Hem-o-lok® clips (Weck Closure System, research Triangle Park, NC, USA) following clear identification of the left ureter laterally. The IMV is identified by dissecting towards the ligament of Treitz and divided at the inferior border of the pancreas. Dissection then continues under the left mesocolon towards the splenic flexure if this has not been completed laparoscopically. During splenic flexure dissection only robot arms 1 and 3 are used to minimise external collisions.
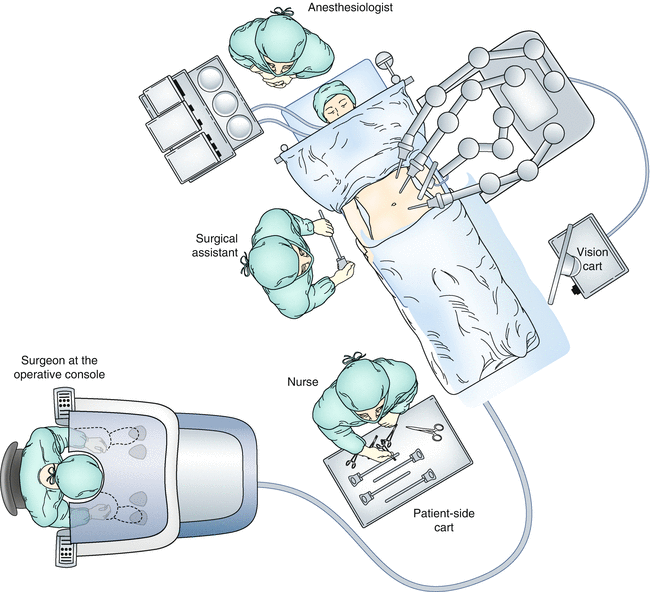

Fig. 14.6
An overhead view of the operating room configuration for robotic low anterior resection (From Choi et al. [23])
For the pelvic dissection the robotic arms are redocked, robotic arm 2 moving to the left lower quadrant port with Cadiere forceps and robotic arm 3 to the left upper quadrant port with the bipolar forceps. The RUQ port or a further 12 mm suprapubic port can also be useful here to aid in rectal retraction.
The robotic Cadiere retracts the rectum anteriorly and the posterior mesorectal plane is dissected as far distally as possible with monopolar scissors. Lateral dissection is then performed, care being taken to preserve the inferior hypogastric nerves. Using the Cadiere to retract the vagina/prostate upwards facilitates anterior dissection, with robot arm 3 being used to provide downwards traction on the rectum (Fig. 14.7). When TME has been performed to the pelvic floor, rectal transection is performed with a laparoscopic stapler, often best accomplished with the robotic cart undocked. When the distal rectum has been divided. The robot is undocked and the rectum extracted via a small suprapubic or left iliac fossa incision with the aid of a plastic wound protector. The specimen is resected, the proximal bowel prepared, and the stapler anvil placed with the aid of a purse string suture. The bowel is returned to the abdomen and the wound either closed or wound protector occluded with a glove placed over the top or clamped prior to re-establishing pneumoperitoneum and forming a stapled anastomosis with laparoscopic assistance.
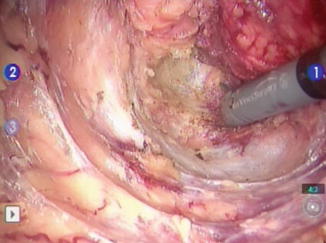

Fig. 14.7
Posterior mesorectal dissection with the Da Vinci robot
Distal resection with the robot remains difficult. Obtaining a right-angled transection of the rectum following TME remains challenging. The options include using conventional laparoscopic stapling instruments or converting to a mini Pfannensteil incision to enable use of an open stapler. A stapler for robotic –assisted surgery is currently becoming available, however, it remains difficult to completely transect the rectum with one firing and an association between number of stapler firings and an increased incidence of anastomotic leak has been identified [26].
Another technique involves division of the rectum and placement of a purse string suture robotically to close the rectal stump and enable single-stapled anastomosis [25].
Other novel techniques have been described to complete a robotic TME. One; robotic assisted transanal surgery for total mesorectal excision (RATS-TME) uses a commercially available GelPOINT Path Transanal Access Platform (Applied Medical, Inc., Rancho Santa Margarita CA, USA) [29]. Following the abdominal component of the procedure, the rectum is divided at the dentate line, the device is placed into the anal canal, insufflation established and the robotic arms redocked. Dissection is carried out in a cephalad direction circumferentially in the mesorectal plane to meet the dissection plane established previously from above.
Learning Curve
As well as skill in manipulating the robot instruments and camera the surgeon has to adapt to the decreased haptic feedback when performing grasping tasks. Also there is the issue of ‘patient side competency.’ Port placement is important to minimise arm clashing during dissection and manipulation and an appreciation of how the robotic arms move for a given input adds to ease in manipulating the bowel around the abdominal cavity.
Virtual reality trainers have been developed with the aim of enhancing learning of robot specific tasks. These have been shown to increase the rate of acquisition of new skills, and could lead to an earlier plateau in the learning curve [30], but also, when used as a warm-up, reduced errors when performing a more complex robotic task [31].
D’Annibale et al. found a decreasing trend in operating time over their first 22 cases and a statistically different operating time between cases 1–22 and cases 40–50 (p = 0.002) [32]. Studies specifically investigating the learning curve indicate that it appears to be overcome after about 15–30 cases [33–36]. This is shorter than that seen with laparoscopic rectal surgery, and there is evidence that it is shorted by previous laparoscopic experience [37].
Perceived Benefits
Current data supports robotic proctectomy as being as oncologically effective and safe as a laparoscopic approach, but there is a lack of high quality evidence.
Meta analysis does describe a longer operating time for robotic TME, however this can vary widely across studies if a hybrid approach is used to perform part of the colonic mobilisation laparoscopically rather than a totally robotic procedure. While all robotic procedures require some time to dock the patient-side cart, this does not appear to be a major influence on operative times, averaging less than 10 min across studies [38, 39].
Oncologic Outcomes
Only one randomized trial has been reported to date [40]. This compared 36 patients assigned to either a robotic or laparoscopic approach. No differences were observed in operative times (p = 0.477) or in quality of mesorectal dissection (p = 0.323). The TME specimen was graded as complete in 17 robotic cases and nearly complete in 1, compared with 13 complete specimens in the laparoscopic group and nearly complete in 3. The 2 converted cases were graded as nearly complete.
There was a non-significant difference in conversion rate (two laparoscopic, nil robotic, p = 0.486) and a significantly shorted length of hospital stay for robotic patients (robotic-assisted, 6.9 ± 1.3 days; standard laparoscopic, 8.7 ± 1.3 days; p < 0.001).
Quality of Dissection
One of the assumptions made of robotic proctec-tomy is that it allows greater operative precision, especially when performing a mesorectal dissection. Studies comparing the CRM between robotic and laparoscopic groups have found no significant difference in specimen quality (Fig. 14.8). A number of studies have examined the TME grade of RALS rectal surgery. Luca et al. graded quality of TME in 28 patients and found 22 complete, 6 nearly complete and 0 incomplete [41]. Baik et al. compared TME grade between RALS and Lap LAR and found a significantly more complete TME specimen in the RALS group (p = 0.03) [42]. Karahasanoglu et al. found the grade of the TME specimen was complete in all 22 rectal RALS specimens [28].
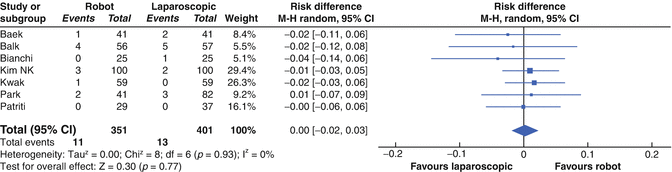

Fig. 14.8
Forest plot showing a meta-analysis of CRM status for rectal RALS vs. CLS. Risk differences are shown with 95 % CIs (From Memon et al. [44])
D’Annibale reports a CRM of <2 mm in 6 of the laparoscopic patients and none of the robotic patients in his series of his first 50 robotic proctectomies compared with 50 laparoscopic proctectomies [32].
Kang et al., in their cohort of 165 patients, having either open, laparoscopic, or robotic surgery for low rectal cancer, found a significantly different rate of CRM involvement for open vs. robotic cases (17, 10.3 % vs. 7, 4.2 %, p = 0.034), but no significant difference in CRM involvement between either open and laparoscopic cases (11, 6.7 %) or laparoscopic and robotic cases [43].
Other indicators of a quality oncological resection such as number of lymph nodes har-vested or distal resection margin have shown no significant difference between laparoscopic and robotic approaches [44].
Surgeon Fatigue
The ergonomic position of the surgeon at the robot console has been thought to be a major advantage to performing precise surgery, especially when compared to the alternative laparoscopic approach, standing beside the patient looking at a remote 2-dimensional monitor. In a laboratory model, robot-assisted surgery appears less physically stressful than standard laparoscopy with both the perception of effort and physical workload being less for similar mental stress [45]. One study that investigated operator and assistant fatigue by Pigazzi et al. asked surgeons to categorically self-report fatigue levels for 6 RALS and 6 CLS low anterior resections (LARs) [46]. 5/6 RALS cases and 2/6 CLS cases resulted in mild fatigue, 1/6 RALS cases and 3/6 CLS cases resulted in moderate fatigue and 1/6 CLS cases resulted in severe fatigue.
Conversion Rates
Conversion rate can be taken as a marker of procedure difficulty with a low conversion rate implying an easier operation. There is evidence that this is significantly lower for robotic proctectomy.
Pratiti et al. reported a conversion rate of 19 % in their laparoscopic group compared to none in the robotic group in spite of the majority of the robotic patients having had previous abdominal surgery and low cancers requiring complete TME [33].
In the metanalysis by Memon et al. (Fig. 14.9), a reduction in risk of conversion of 7 % (95 % CI 1–12) was found, however there appeared to be marked differences between studies, due to the small numbers in each.
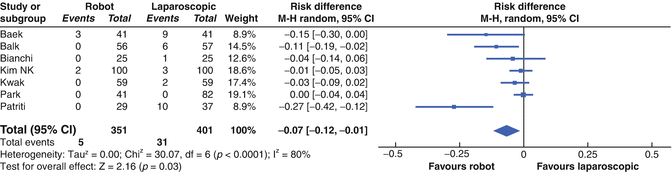

Fig. 14.9




Forest plot showing a meta-analysis of conversion rates for rectal RALS versus CLS. Risk differences are shown with 95 % CIs (From Memon et al. [44])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






