Chapter 3.15
Lactose malabsorption and nutrition
Pascale Gerbault, Anke Liebert, Dallas M. Swallow and Mark G. Thomas
University College London, London, UK
The consumption of milk and dairy products varies considerably in different regions of the world. Indeed, according to the statistics of the Food and Agriculture Organization of the United Nations (FAO), in 2007 the consumption of milk and dairy products averaged 240 kg and 360 kg per capita in the UK and Sweden, respectively, while in China it was about 29 kg per capita. Milk is a complex and nutrient-dense food [1] that may have positive or negative effects on adult health [2]. The major carbohydrate component in milk is lactose, a disaccharide whose concentration in bovine milk has been reported to range between 45 and 55 g/L [2–4]. Lactose needs to be digested by the small intestinal enzyme lactase into its constituent monosaccharides, glucose and galactose, before transport across the epithelial cell membranes.
Lactase activity is therefore essential for the development of young mammals, since their sole source of nourishment is their mother’s milk. In most mammals, including most humans, lactase expression decreases after the weaning period is over [5]. In humans, this condition is termed lactase non-persistence and is observed in around 65% of adults worldwide [6,7]. Lactase non-persistent individuals are sometimes described as having primary adult hypolactasia and are lactose maldigesters, while adults who have the genetically determined trait of lactase persistence (LP) and continue to produce lactase throughout life are termed lactose digesters.
The range of timing of lactase downregulation varies from one population to another; for example, most Chinese and Japanese become lactase non-persistent between 1 and 5 years old, while on average lactase non-persistence does not manifest in Finns and Estonians until somewhat later [8]. Even though the mechanisms of developmental lactase downregulation are not well understood, it is clear that it is not reversible [9,10]. Lactase production can also be lost through non-genetic mechanisms; this is called secondary hypolactasia and it can occur, for example, after any condition that damages the small intestinal mucosa brush border [11]. Adults with either primary or secondary hypolactasia are lactose malabsorbers and may exhibit symptoms of lactose intolerance after ingestion of lactose. Genetically determined lactase non-persistence is quite normal in the majority of humans worldwide and is distinct from congenital alactasia, the absence of lactase from birth. This, in contrast, is an extremely rare and potentially fatal condition. A number of mutations that affect the structure of the protein, and consequently its function, have been identified in Finnish patients suffering from this condition [12,13].
Two types of tests are available for determining lactase production status at the phenotypic level. Duodenal or jejunal biopsies can be taken by endoscopy and allow direct determination of lactase activity. A lactase assay is usually combined with routine histology and an assay of another enzyme such as sucrase, so that secondary deficiency of lactase can be readily identified. This procedure is the most accurate available, but it is invasive and performed routinely only if a pathological condition such as coeliac disease is indicated. Other methods involve lactose ingestion after an overnight fast to inform indirectly on lactase activity [14]. For the glucose test, an increase in blood glucose is indicative of LP as lactase cleaves lactose into glucose and galactose. Although less commonly used for identifying LP, a urinary galactose test can also be performed, which also involves giving alcohol to block galactose uptake by the liver. Alternatively, the breath hydrogen test measures hydrogen production by colonic bacteria; in lactase non-persistent individuals, undigested lactose reaches the colon and hydrogen is released after fermentation by hydrogen-producing colonic bacteria, while in persistent individuals lactose is cleaved before reaching the colon. These tests require a baseline measurement of glucose, galactose or breath hydrogen before ingestion of the lactose load, and further measurements of the same at about 30-min intervals for 2–3 h. It should be noted that these indirect tests are not 100% accurate (error rates are discussed in Mulcare et al. [15]) and cannot distinguish primary from secondary hypolactasia. For example, there are some individuals who do not have colonic bacteria that produce hydrogen, and therefore do not show a hydrogen rise irrespective of their lactase production status.
The passage of lactose into the colon in non-lactase producers can lead to GI symptoms, such as bloating, flatulence, abdominal pain and diarrhoea. These symptoms are described as ‘lactose intolerance’. Lactose intolerance should be distinguished from milk allergy; while the former is a non-toxic and non-immune adverse reaction to undigested lactose, milk allergy involves an immune response, usually to milk protein.
After an individual has been diagnosed as lactase non-persistent, dairy products are often removed from the diet. This may have serious nutritional disadvantages, such as reducing the intake of calcium, phosphorus and vitamins, and may be associated with decreased bone mineral density [2,16,17], while lactose-free milk products intake would avoid symptoms and be nutritious. In contrast, milk powder (which does contain lactose) has been used for famine relief in undernourished populations where the frequency of LP is often very low and for whom symptoms of intolerance can potentially exacerbate diarrhoeal disease and mineral deficiency [18,20,21].
3.15.1 Lactase persistence
This section briefly summarises what is known about the genetics and evolution of the LP trait, but further details and references can be found in Gerbault et al. [22] and on the global lactase persistence database website (www.ucl.ac.uk/mace-lab/resources/glad).
The global distribution of lactase persistence
Figure 3.15.1 shows an interpolated map of the frequency of LP in indigenous populations worldwide. LP is particularly common in northern Europe, with frequencies of around 89–96% in the British Isles and southern Scandinavia; a declining gradient towards the south and east is seen in the rest of Europe. On other continents LP is not evenly distributed geographically. Indeed, in Africa and the Middle East it is often found at very different frequencies in neighbouring populations, such as 64% in the Beni Amir (pastoralists) and 23% in the Dounglawi (non-pastoralists) in Sudan. LP frequency has been shown to correlate strongly with a tradition of pastoralism [23].
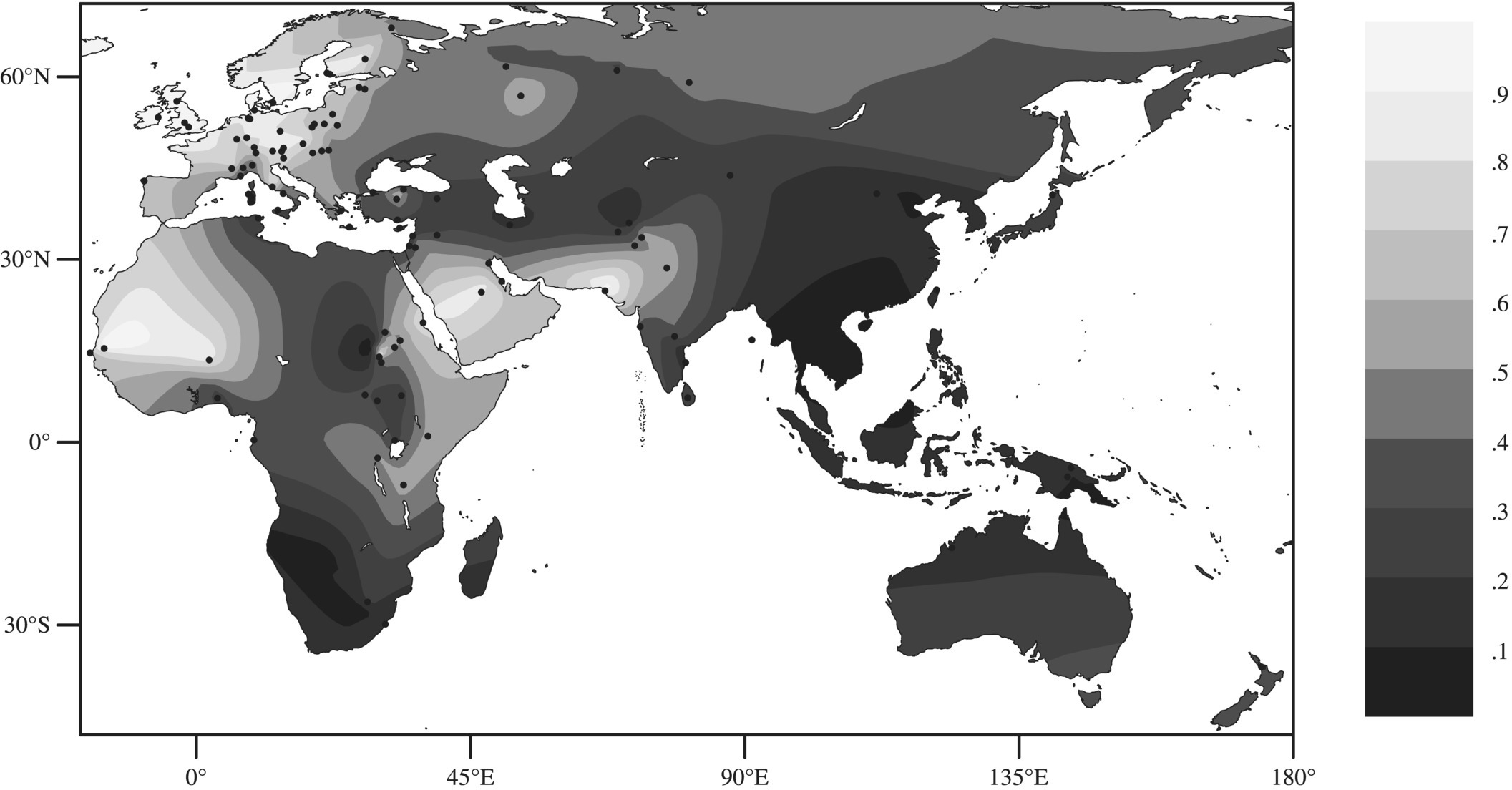
Figure 3.15.1 Interpolated map of LP phenotype distribution in the ‘Old World’. Data points (dots) were taken from the literature (for details see Ingram et al. [6]). The key shows the frequency of the LP phenotype, black being used for the lowest frequency while white is used for the highest. Source: Itan et al. [7]. Reproduced with permission from BioMed Central Ltd.
The genetics of lactase persistence
Lactase persistence is inherited in an autosomal dominant manner. A single gene (LCT) codes for lactase. Several single nucleotide changes have been found in a lactase gene regulatory region (so-called enhancer, which is located in the adjacent gene MCM6), one of which occurs at high frequency in Europe. Estimates of the age of these changes range between 2,188 and 20,650 years ago [24]and between 7,450 and 12,300 years ago [25] for the −13,910*T allele associated with LP in Europe and southern Asia, and between 1200 and 23,200 years ago for the −14,010*C allele, one of the major LP-associated variants in Africa [26]. These date estimates bracket those for the domestication of milkable animals and the spread of agriculture and herding obtained from archaeological data (cave paintings, distributions of animal bones and dairy fat residues in pots).
Natural selection and evolution of lactase persistence in humans
A low frequency or absence of −13,910*T in early Neolithic central European farmers [27] and early Neolithic farmers from north-east Iberia [28], middle Neolithic Scandinavian hunter-gatherers [29] and late Neolithic farmers from southern France [30] suggests that dairying was practised before LP arose or became common. The age estimates for the LP-associated variants are remarkably young for alleles found at such high frequencies in multiple populations, suggesting that their spread has been boosted by natural selection. The strength of natural selection estimated for the LP-associated alleles is very high (1.4–19% [23] and 5.2–15.9% [31] for −13,910*T, and 1–15% for −14,010*C [26]), amongst the highest for any human genes in the last 30,000 years.
Several lines of evidence (genetics, anthropology and archaeology) suggest that LP would not have provided a selective advantage without a supply of dairy products containing lactose to adults, implying that these traits evolved as the result of a co-evolutionary process involving both genes and culture. A spatially-explicit computer simulation study of this gene–culture co-evolutionary process in Europe indicate that LP and dairying began between 6856 and 8283 years ago in a region around modern-day Hungary [31].
3.15.2 Implications for diet today
Variable symptoms of hypolactasia in adults
Symptoms of lactose intolerance can arise after an individual with hypolactasia has ingested lactose. Firstly, when undigested lactose passes into the colon it creates an osmotic gradient across the GI wall, driving an influx of water to re-equilibrate the osmotic imbalance, which can lead to diarrhoea. Secondly, the fermentation of lactose by colonic bacteria can lead to the production of fatty acids and various gases as by-products (including hydrogen), potentially causing discomfort, bloating and flatulence (reviewed in (Hammer et al. [32]). These symptoms usually manifest within 1–2 h of ingestion, but vary greatly from one individual to another.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





