Kidney and Pancreas Transplantation in the Diabetic Patient
Gerald S. Lipshutz
Diabetes mellitus is a major health problem worldwide, affecting as many as 135 million people. In the United States, it affects about 6% of the population (18 million individuals), with at least half being unaware that they have the disease. It accounts for more than 160,000 deaths each year in the United States, and in 2002, the annual direct and indirect costs of type 1 and 2 diabetes exceeded $130 billion. The prevalence of type 1 diabetes in the United States is estimated to be 1,000,000 people, with 30,000 new cases diagnosed each year, and this has not substantially changed in the recent past.
At the turn of the 19th century, a patient diagnosed with type 1 diabetes had an average life expectancy of 2 years. However, with the isolation and development of insulin as a treatment for diabetes, the disease has been changed from one that is rapidly fatal to a chronic disease with the potential for multiple secondary complications within 10 to 20 years after disease diagnosis. These include blindness, cardiovascular disease, dyslipidemia, cerebrovascular disease, amputation, and life-span reduction.
Diabetes mellitus is the leading cause of end-stage renal disease (ESRD), accounting for about one third of new dialysis-dependent patients each year. About 40% of the ESRD population has diabetes, and most have type 2 diabetes. The incidence of ESRD as a consequence of type 2 diabetes is increasing in all countries with a Western diet and lifestyle. Because of the growth in diabetes in the population, the number of diabetic patients with new ESRD has surpassed the number of patients with ESRD from all other primary diagnoses and most commonly leads to kidney transplantation in adult whites, Asians, and Native Americans. In addition to ESRD, major complications in these patients include retinopathy, which is the second leading cause of blindness in all persons, and peripheral vascular disease. Ten percent of diabetic patients require a major amputation in their lifetime. Life expectancy is about one third lower in diabetic patients compared with nondiabetic patients, and cardiovascular disease is the leading cause of death.
For many patients with type 1 diabetes mellitus, the treatment of choice is a whole vascularized pancreas transplantation. As of the end of 2004, more than 23,000 pancreas transplantations had been performed worldwide, and through 2006, more than 20,000 pancreas transplantations were performed in the United States. Since 2000, the 1-year patient survival rate for simultaneous pancreas and kidney (SPK) transplantation, pancreas after kidney (PAK) transplantation, and pancreas transplantation alone (PTA) were 95% to 97%, and the 1-year pancreas graft survival rates were 85%, 78%, and 77%, respectively.
The potential benefits of a pancreas and kidney transplantation in a patient with type 1 diabetes and renal failure are improved quality of life, prevention of recurrent diabetic nephropathy, freedom from exogenous insulin with euglycemia and normalization of glycosylated hemoglobin, lack of frequent whole blood glucose monitoring, lack of dietary restrictions, and stabilization or improvement in secondary complications. The benefits of an SPK are the basis of its acceptance as an appropriate therapy for patients with type 1 diabetes mellitus and renal failure. The tradeoff for the patient is the operative risk
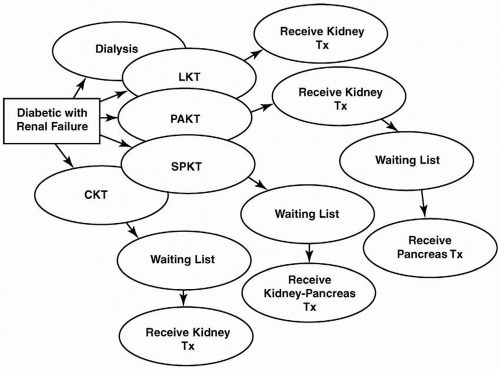
and the need for lifelong chronic immunosuppression. Pancreas transplantation is the ultimate intensification of insulin therapy because it normalizes glucose levels far better than any other strategy available for the treatment of type 1 diabetes mellitus. In this chapter, the issues concerning pancreas transplantation in type 1 diabetic patients are presented. The indications for, technical differences between, and management of the different methods of pancreas transplantation are discussed. The special concerns regarding kidney transplantation in both type 1 and type 2 diabetic patients are discussed in Chapters 7 and 10. Figure 15.1 illustrates the whole-organ transplantation options available for type 1 diabetic patients with advanced renal disease and ESRD.
and the need for lifelong chronic immunosuppression. Pancreas transplantation is the ultimate intensification of insulin therapy because it normalizes glucose levels far better than any other strategy available for the treatment of type 1 diabetes mellitus. In this chapter, the issues concerning pancreas transplantation in type 1 diabetic patients are presented. The indications for, technical differences between, and management of the different methods of pancreas transplantation are discussed. The special concerns regarding kidney transplantation in both type 1 and type 2 diabetic patients are discussed in Chapters 7 and 10. Figure 15.1 illustrates the whole-organ transplantation options available for type 1 diabetic patients with advanced renal disease and ESRD.
HISTORY OF PANCREAS TRANSPLANTATION
The first human pancreas transplantation was performed in 1966 by William Kelly and Richard Lillehei at the University of Minnesota. The major surgical challenge that needed to be overcome was a method of pancreatic exocrine drainage. A duct-ligated segmental pancreatic allograft and a deceased donor kidney were transplanted into a 28-year-old woman with type 1 diabetes mellitus and ESRD. Post-transplantation immunosuppression was azathioprine and prednisone. A pancreatic fistula complicated the patient’s postoperative course, and both the kidney and pancreas were removed about 2 months later. Subsequently, the patient died from a pulmonary embolus. The second patient, a 32-year-old recipient, was transplanted 2 weeks after the first recipient. The
patient suffered from rejection and was treated with steroid boluses and graft irradiation. The patient died from sepsis 41/2 months after transplantation.
patient suffered from rejection and was treated with steroid boluses and graft irradiation. The patient died from sepsis 41/2 months after transplantation.
Although these initial results were individually poor, they were at the same time encouraging in that these early transplantations did demonstrate that glucose control without exogenous insulin was possible. The procedure established that endogenous secretion of insulin with normal feedback mechanisms could occur with a whole-organ vascularized pancreas transplantation.
SURGICAL OPTIONS FOR DIABETIC PATIENTS
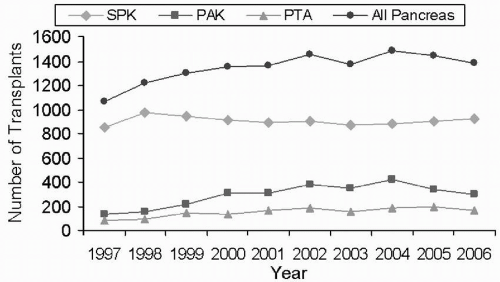
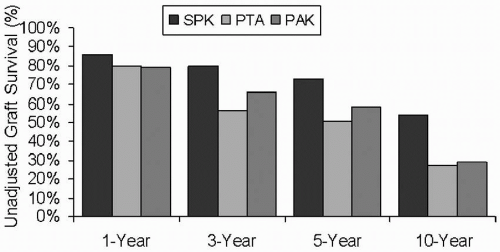
There are three major procedures for type 1 diabetic patients who are considering whole-organ pancreas transplantation. First is the SPK transplantation for diabetic patients with advanced renal disease or ESRD. A major advantage of SPK transplantation is that there is only one surgical intervention and one source of foreign human leukocyte antigen (HLA) to which the patient is exposed. The largest numbers of pancreas transplantations are performed as SPK transplantations (Fig. 15.2). Chronic immunosuppressive therapy is similar to that which these patients would receive with a kidney transplant alone. As in PAK transplantation, however, many patients have already suffered substantial secondary diabetic complications, and the extent to which these complications will reverse or stabilize is uncertain. Regardless, SPK transplantation is established as a therapeutic and effective procedure, and not only is it lifesaving for the type 1 diabetic patient, but also numerous studies have demonstrated that it is life enhancing, with a significant overall improvement in quality of life indices over kidney transplantation alone. Among pancreas recipients, those with an SPK transplantation had the best pancreas graft survival rates: 86% at 1 year and 54% at 10 years (Figs. 15.3 and 15.4).
Second is PAK transplantation for the diabetic patient who is already the recipient of a functioning kidney allograft. Immunosuppressive therapy is not the major concern because these patients are already receiving chronic
immunosuppression. The main risk to the patient is the alteration in immunosuppression necessary after pancreas transplantation. This alteration can negatively affect postoperative renal function because immunosuppressant levels are often increased in these recipients compared with renal transplant alone recipients. In addition, there is the inherent risk for an intra-abdominal surgical procedure. PAK transplantation may be a particularly important option for patients with a living donor, in which case the kidney is placed in the left iliac fossa in anticipation of pancreas transplantation in the future. The graft survival rate for PAK recipients at 1 year is 79%, with a 10-year survival rate of 29% (Fig 15.3).
immunosuppression. The main risk to the patient is the alteration in immunosuppression necessary after pancreas transplantation. This alteration can negatively affect postoperative renal function because immunosuppressant levels are often increased in these recipients compared with renal transplant alone recipients. In addition, there is the inherent risk for an intra-abdominal surgical procedure. PAK transplantation may be a particularly important option for patients with a living donor, in which case the kidney is placed in the left iliac fossa in anticipation of pancreas transplantation in the future. The graft survival rate for PAK recipients at 1 year is 79%, with a 10-year survival rate of 29% (Fig 15.3).
PAK recipients have already suffered significant secondary diabetic complications. Other than making these recipients insulin independent, it is uncertain whether a well-functioning pancreatic allograft will have any additional benefit in the long-term. Overall, the results of PAK transplantation are worse than those of SPK transplantation, likely related to difficulties in diagnosing pancreatic allograft rejection because the kidney (owing to differing HLA) is now longer available as surrogate to assess for rejection by biopsy. It is the second most common pancreas transplant operation. In 1999, Medicare-approved reimbursement for pancreas transplantation for patients with ESRD (i.e., those receiving an SPK and PAK but not PTA), making the procedure available for a much larger population of patients.
The third method, and by far the least common, is pancreas transplantation alone (or PTA) in the preuremic recipient. This is a therapeutic option for diabetic patients with minimal to no renal dysfunction who have brittle diabetic control despite the administration of insulin. Many also have hypoglycemic unawareness. The main risks to these patients are the long-term effects of chronic immunosuppression, not only on native renal function but also in the development of atherosclerosis and increased risk for malignancy, and the surgical procedure itself. The number of patients receiving these transplants has increased, likely due in part to improvements in immunosuppression. In 2004, less than 250 patients underwent PTA in the United States. One-year graft survival rates for PTA recipients is 80%, with a 10-year rate of 27% (Fig 15.3). The American Diabetes Association (ADA) criteria for PTA are as follows:
▪ Consistent failure of intensive insulin-based therapy to establish reasonable glycemic control and to prevent secondary complications
▪ Incapacitating clinical and emotional problems with exogenous insulin therapy
There is controversy regarding the survival benefit with PTA because of its associated morbidity and mortality, the need for immunosuppression, and questions about whether secondary complications are prevented. Most centers only consider the procedure in diabetic patients with severe hypoglycemic unawareness or significant secondary complications of diabetes without renal dysfunction. The option of islet transplantation on these patients is discussed later.
EVALUATION OF THE PANCREAS TRANSPLANTATION CANDIDATE
Recipient selection and pretransplantation evaluation are essential to avoid significant transplant-related complications. Waitlist candidates should be seen and examined routinely while awaiting organ transplantation. Specific studies should be repeated if the patient remains on the waitlist for a prolonged period of time.
Coronary Artery Disease
Serious vascular complications limit the success of transplantation in diabetic patients. These patients often have multiple cardiovascular risk factors in addition to the long history of diabetes mellitus. These often include tobacco use,
hypertension, hyperlipidemia, family history, and renal failure. Nearly half of diabetic transplant recipients die within 3 years after transplantation from a vascular complication, and in pancreas transplant recipients, cardiovascular disease is the single greatest cause of death. Type 1 diabetes patients are at particularly high risk for premature coronary atherosclerotic disease, with as many as 35% dying of coronary artery disease by age 55 years. Coronary artery disease prevalence increases significantly with age and has been found in most patients older than 45 years. The risk for death in these patients is increased 8- to 15-fold in patients with nephropathy.
hypertension, hyperlipidemia, family history, and renal failure. Nearly half of diabetic transplant recipients die within 3 years after transplantation from a vascular complication, and in pancreas transplant recipients, cardiovascular disease is the single greatest cause of death. Type 1 diabetes patients are at particularly high risk for premature coronary atherosclerotic disease, with as many as 35% dying of coronary artery disease by age 55 years. Coronary artery disease prevalence increases significantly with age and has been found in most patients older than 45 years. The risk for death in these patients is increased 8- to 15-fold in patients with nephropathy.
Most of these patients do not suffer typical anginal symptoms, and thus the possibility of unknown coronary artery disease should be considered in every diabetic patient being considered for organ transplantation. All patients should undergo appropriate evaluation preoperatively according to general recommendations documented in Chapter 7. The precise protocol used is controversial and center specific. The controversy is likely related to the poor predictive value of noninvasive imaging in diabetic transplant candidates. Nuclear perfusion imaging is best performed as a screening study. In general, young patients who have had diabetes mellitus for less than 25 years, have not smoked tobacco, and lack other cardiovascular risk factors may be evaluated by stress imaging alone. A treadmill nuclear stress test used with thallium or sestamibi scintigraphy or echocardiography is an appropriate initial study. Many diabetic patients with ESRD, however, have poor exercise tolerance and are unable to obtain a rate of 85% of their predicated maximal heart rate. These patients should undergo an adenosine nuclear stress test or a dobutamine stress echocardiogram designed to replicate the effect of exercise stressing on cardiac function.
Most other candidates should be evaluated by coronary angiography, and significant coronary artery disease should be appropriately treated before undergoing transplantation. In addition, patients are generally recommended to undergo routine annual reassessment with noninvasive stress imaging until transplanted, although the benefit of this commonly used strategy had not been prospectively documented. The fact that these patients have had longstanding diabetes should not be forgotten after a successful pancreas transplantation. Risk factor modification should continue throughout the pretransplantation and post-transplantation periods.
Pancreas transplant candidates with multiple risk factors or a positive nuclear stress test should undergo cardiac angiography and evaluation by a cardiologist before candidacy determination. Patients with coronary lesions amenable to bypass grafting or angioplasty and stent placement should be treated before transplantation. If patients require a postprocedure course of clopidogrel bisulfate, it is preferable that this be completed before undergoing transplantation. However, patients with significant coronary artery disease that is not amenable to interventional cardiology or surgical therapy should not be considered candidates for pancreas transplantation.
Aggressive risk factor modification including statins for elevated lowdensity lipoprotein cholesterol and total cholesterol should be instituted. When possible, patients should be started on low-dose β blockade if they do not have hypoglycemic unawareness of other contraindications. β1-Selective blockers are preferable to avoid undesirable side effects. Antihypertensive drugs that do not aggravate insulin sensitivity or lipid metabolism should be selected for treatment of arterial hypertension. β1 Blockers without intrinsic sympathomimetic action are preferable for patients with both diabetes and hypertension and with associated ischemic heart disease, whereas β1 blockers with intrinsic sympathomimetic action, exerting a vasodilative action, are useful for diabetic hypertensive patients without ischemic heart disease because they do not aggravate
insulin sensitivity and lipid metabolism. In addition, daily aspirin and omega-3 and omega-6 fatty acids should be recommended to promote vascular health.
insulin sensitivity and lipid metabolism. In addition, daily aspirin and omega-3 and omega-6 fatty acids should be recommended to promote vascular health.
Cerebrovascular and Peripheral Vascular Disease
The increased susceptibility of diabetic transplant recipients for cerebrovascular and peripheral vascular disease mandates particular attention to these issues in the pretransplantation evaluation. About 4% of kidney alone and SPK recipients experience a stroke or transient ischemic attack in the 4-year postoperative evaluation period; nearly one third of these are fatal. Any history of cerebrovascular events or intermittent claudication or findings of carotid or femoral bruits or poor peripheral pulses should be further assessed during patient evaluation. Further consultation with a vascular surgeon may be necessary.
Infections
Patients should be free of significant infections, such as peritonitis, osteomyelitis, or unhealed foot or lower extremity ulcerations at the time of transplantation. Close examination of the patient’s feet and lower extremities should be performed at each visit and on admission for organ transplantation. If a patient is admitted to undergo transplantation and a lower extremity ulcer is found, that patient should be discharged and should notify the transplantation center when it is completely healed. Significant dental decay and periodontal disease should also be treated before transplantation. Patients should be informed that if they develop infectious complications while awaiting transplantation, their candidacy will be placed “on hold” until all infectious issues have been resolved.
Predialysis Transplantation
The advantages of predialysis transplantation for kidney transplant candidates are discussed in Chapter 7; they also apply to candidates for SPK. Early transplantation can obviate the need for both temporary and permanent dialysis access and disfigurement of the extremities associated with these procedures, can prevent episodes of congestive heart failure and fluid overload, and can correct hypertension, which may contribute to more rapid vision loss in this group of patients. Some data suggest that early transplantation may slow retinopathy and correct neuropathy. The development of diabetic complications on dialysis may impair the rehabilitation potential after transplantation.
Predialysis diabetic transplant candidates who require coronary angiography risk worsening of renal function and potential dialysis initiation induced by exposure to iodinated contrast agents. This risk of contrast-induced nephropathy has to be carefully weighed against the risks associated with undiagnosed coronary artery disease. Working closely with a cardiologist can be helpful in that the dose of intravenous contrast administered during coronary angiography can be minimized to reduce the risk for precipitating renal failure.
Insulin Requirements
By the time many diabetic patients develop advanced nephropathy or the need for dialysis, their insulin requirements have often diminished. Patients receiving peritoneal dialysis may have higher insulin needs owing to the use of dextrose containing dialysates. Pancreas transplant candidates should have a C-peptide level drawn to confirm they are insulinopenic; however, their history will likely confirm their diagnosis. In type 1 patients a C-peptide value should be undetectable, or less than 0.5 ng/mL. Although several centers do perform pancreas transplantation in insulinopenic type 2 diabetic patients, this has not been widely adopted.
It may be more difficult to achieve adequate postoperative insulin levels in recipients who have a daily insulin requirement of greater than 60 units.
Obese type 1 diabetic patients may have also developed insulin resistance, and an estimate of the pretransplantation insulin requirement may be helpful in assessing the need for exogenous insulin after transplantation. Some glucose intolerance can be seen in the early postoperative period owing to large doses of corticosteroids, carbohydrate intolerance, infusion of medications prepared in 5% dextrose, improved appetite, and the use of calcineurin inhibitors that may lead to periods of elevated blood glucose and increased insulin requirements. Type 1 diabetic patients should expect to be free of exogenously administered insulin after a successful transplantation.
Obese type 1 diabetic patients may have also developed insulin resistance, and an estimate of the pretransplantation insulin requirement may be helpful in assessing the need for exogenous insulin after transplantation. Some glucose intolerance can be seen in the early postoperative period owing to large doses of corticosteroids, carbohydrate intolerance, infusion of medications prepared in 5% dextrose, improved appetite, and the use of calcineurin inhibitors that may lead to periods of elevated blood glucose and increased insulin requirements. Type 1 diabetic patients should expect to be free of exogenously administered insulin after a successful transplantation.
DONOR SELECTION
Appropriate pancreas donor selection is key to avoiding complications relating primarily to vascular thrombosis and duodenal enteric leaks. The organ donor for pancreas transplantation is typically in the age range of 10 to 45 years with a traumatic mechanism as the cause of brain death. Donors whose death is defined by cardiac criteria (see Chapter 4) are not suitable for whole-organ pancreas donation. The donor should have had no previous pancreatic surgery or history of pancreatic trauma, or a diagnosis of diabetes mellitus. A HgbAlc level before procurement may help assess for glucose intolerance. Hyperglycemia is a common occurrence during the management of brain dead patients and does not represent a contraindication to pancreas donation.
An increased incidence of allograft thrombosis and graft loss has been described when the donors are aged greater than 45 years or have died from cerebrovascular accidents. Pancreata originating from older donors have had higher rates of intra-abdominal infections, anastomotic or duodenal leaks, relaparotomy, and decreased graft survival. As a result, caution should be urged in accepting and using pancreata from organ donors older than 45 years. Weight and body mass are also important considerations. Although no strict criteria exist regarding donor weight, some centers consider a donor lower weight limit of 45 kg. This is primarily because of concern of the size of the pancreatic arterial vasculature for construction of the iliac Y-graft and risk for arterial graft thrombosis. Some centers, however, routinely use pancreata from small or even pediatric donors with good outcomes. Donors with a body mass index higher than 30 are avoided by many centers because of an increased incidence of fatty infiltration and subsequent increased risk for ischemia-reperfusion injury, infection, pancreatitis, and allograft thrombosis.
PANCREAS TRANSPLANTATION: SURGICAL TECHNIQUE
The surgical procedure can be divided down into three stages: (1) organ procurement, (2) back table pancreas preparation, and (3) pancreas transplantation.
Organ Procurement
Successful and uncomplicated pancreas transplantation requires meticulous allograft procurement and attention to detail in preparing the pancreas on the back table. There is no substitute for a skilled surgeon examining the pancreas during procurement and making an assessment of the suitability of the organ.
After opening the lesser sac, the gastrocolic ligament is divided, and the pancreas is closely examined and palpated. The aorta and venal cava are exposed, followed by division of the right gastroepiploic and pyloric vessels. Some centers perform a bowel decontamination procedure. A nasogastric tube may be advanced into the second portion of the duodenum, and 200 mL of saline and povidone-iodine with amphotericin B is instilled. The short gastrics are ligated, the transverse colon is completely mobilized, and the stomach is then divided proximal to the pylorus. The fourth portion of the duodenum is




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree