Fig. 9.1
Juxtahepatic (or retrohepatic) vena cava is in direct contact with the posterior aspect of the liver
The additional methods available to evaluate the abdomen include focused abdominal sonography for trauma (FAST), computed tomography, and diagnostic peritoneal lavage. FAST has become a highly reliable test used when seeking to determine whether there is blood in the abdomen in a patient who is hemodynamically labile. In the stable patient in whom liver injury is suspected, the use of computed tomography has become widespread. Several classification schemes have been described for liver injuries. There are inconsistencies in the terminology, but a grading scheme proposed by the American Association for the Surgery of Trauma (AAST) is now in wide use (see Table 5.1, page 48).
These diagnostic adjuncts are not intended to replace clinical judgment and examination by the surgeon. Whatever method is used for abdominal evaluation should be readily available, and the surgeon should be proficient in its use and interpretation.
Penetrating abdominal injury presents a more straightforward situation for the evaluating surgeon. What must be remembered is the anatomic position of the liver, under the costal margin, especially as this relates to thoracoabdominal injuries. Hemodynamic lability mandates exploratory celiotomy, irrespective of the mechanism of injury (stab versus gunshot wound).
Approach to the Injured Liver
With hepatic injuries, the paramount decision is to determine if an intervention is needed to control hemorrhage. Hemodynamic instability mandates expeditious operative management or angiography/embolization (if the patient can be stabilized with volume resuscitation) in order to make transportation to the radiographic suite less risky for the patient. The hemodynamically stable patient may be evaluated by any of the methods noted earlier. Minor grade I or II injuries frequently require no operative intervention. When diagnosing by CT scan, the surgeon must be cognizant of the magnitude and anatomy of the liver injury. Contusion contained within the liver capsule or minor laceration, such as in grade I or II injuries, may be observed. These diagnoses together constitute most liver injury cases, accounting for 60 or 70 %. Grade III injuries (deeper, larger wounds with more tissue destruction) occur in approximately 25 % of cases. Grade IV and V injuries, involving large amounts of tissue destruction, have an incidence of 7 and 3 %, respectively, and are highly lethal. It should be emphasized that evidence of blood in the peritoneal pericolic gutters, in the pelvis, or tracking along the periportal triads [1] is suggestive of a more significant injury than the liver anatomy may indicate and mandates exploration. Also, the concomitant existence of hollow viscous injury occurs in 5 and 20 % of major hepatic injuries.
In addition, it is important to realize that massive parenchymal injuries can occur with surprisingly little bleeding and that minor lacerations may bleed profusely. An understanding of the tissue architecture of the liver is a prerequisite to successful management. Most blunt lacerations may occur along segmental fissures, because the vascular and biliary duct structures are moderately shear resistant. This explains why a large stellate or “bear claw” laceration may be seen, with little or no intraperitoneal blood in a hemodynamically stable patient. This can be managed nonoperatively with observation and repeated CT scan. Nonoperative treatment of the stable patient sustaining a blunt liver injury is the management approach of choice today [2–7].
Conversely, the deceleration forces are responsible for a shear effect that can result in avulsion of the hepatic veins from the vena cava or major branches of the portal venous or hepatic venous systems. Hemorrhage is devastating, difficult to control, and responsible for the high mortality rate seen with such injuries.
Penetrating injuries present their own set of difficulties; missile tracts may bleed profusely. The same elasticity that can protect the vascular structures from shear forces has little if any effect when confronted with a missile.
Intraoperative Decisions: General Principles
Once the decision to operate has been made, the surgeon needs to proceed in an orderly fashion, to a fully equipped operative theater, including the capabilities of invasive monitoring. Before opening the abdomen, the surgeons must ensure that there is optimal venous access. Large-bore central access is essential. The prudent, historic dictum is that access should be from the upper torso in the event there is a retrohepatic caval injury. The blood bank must be notified of the potential for massive transfusion of packed cells and blood components to treat the often associated coagulopathy. The development of blood salvage systems has greatly improved the care of these patients; shed blood from the operative field can be washed and reinfused, provided there is no evidence of gastrointestinal contamination. Infusion systems are available that allow rapid delivery of large volumes of warmed fluid to help minimize hypothermia and hypovolemia. Hypothermia is a common cause of coagulopathy and must be aggressively defended against. Invasive monitoring capabilities are essential in the management of these critically injured patients.
Optimal surgical exposure is essential and starts with performing a midline vertical incision for expedient entry into the abdominal cavity. The incision should extend from the xiphoid process to the symphysis pubis. Such an approach allows, if necessary, relatively easy extension into the thorax through either a median sternotomy or a lateral thoracotomy.
Performing a celiotomy could potentially decompress the tamponade, thereby necessitating expeditious vascular control. The need to perform an emergency thoracotomy for vascular control of the aorta before opening the abdomen is rarely indicated. Such control of the bleeding can usually be obtained with the assistant’s manual compression of the liver.
Once the abdomen is open, a rapid assessment of the injury is made and priority management begun. All clot is evacuated, and the four quadrants are packed to control bleeding. A sequential examination is then carried out with priority given to control of blood loss followed by control of any enteric content spillage.
The general approach to the liver injury requires adequate visualization of the anatomic features of the injury. This may require mobilization of the liver along the falciform and triangular ligaments. Full mobilization of the liver allows delivery onto the abdominal wall that can often facilitate suture repair of a hepatic wound in a difficult area. When mobilizing the liver, care must be taken that the hepatic veins are not injured. The coronary ligaments are in close proximity to hepatic veins. Also, the surgeon must be cautious during the mobilization of the liver that venous return through the vena cava is not obstructed for a prolonged period. Hemorrhage control during mobilization can often be done by the assistant’s applying direct pressure with laparotomy packs, compressing the liver between the hands.
As noted previously, most of the injuries encountered are grade I or II and require little more than simple suture repair. Grades III, IV, and V injuries require an organized approach for successful control of hemorrhage, which includes manual compression, direct ligation, or clipping of lacerated vessels, along with sophisticated techniques for more complex wounds.
Vascular occlusion of the portal triad (performing the Pringle maneuver) is a useful method of controlling hepatic arterial and portal venous inflow to the liver. A noncrushing or vascular clamp can be applied to the porta hepatis and safely left in place for approximately 45 min, although the specific duration threshold is not known for the hemodynamically labile patient. Also, an umbilical tape placed around the porta hepatis structures can be used for such control. If this maneuver markedly reduces the liver’s bleeding, the parenchymal injury can be assessed and a method of repair decided on. However, if hemorrhage persists, then an intrahepatic portal vein injury or a major hepatic vein injury must be suspected.
Proceeding with hepatotomy for localization and control of hemorrhage requires fastidious cooperation between the surgeon and the first assistant. With the depth of the hepatic wound exposed, it is usually the first assistant who controls the bleeding. The finger-fracture technique for hepatotomy, with the first assistant compressing the liver, is very effective. The operating surgeon using the fingertips or the handle of a scalpel to separate the liver parenchyma and the assistant using a multiple loaded clip applier, made popular for laparoscopic cholecystectomy, ligate severed vessels. Nonabsorbable suture ligation can also be performed to control vessels as each is encountered. Knowledge of the anatomy of the liver (along with reported anatomic variants) is a prerequisite to this approach. The confluence of the left and middle hepatic veins must be kept in mind to avoid overzealous ligation (Fig. 9.2). Likewise, the position of the inferior vena cava and the hepatic veins to the caudate lobe should be noted to avoid unnecessary injury that may complicate the surgical management. The placement of random deep sutures is fraught with difficulty. Failure of the abovementioned maneuvers to control hepatic bleeding means that the surgeon either has not adequately identified the source or is dealing with coagulopathic bleeding (or both).
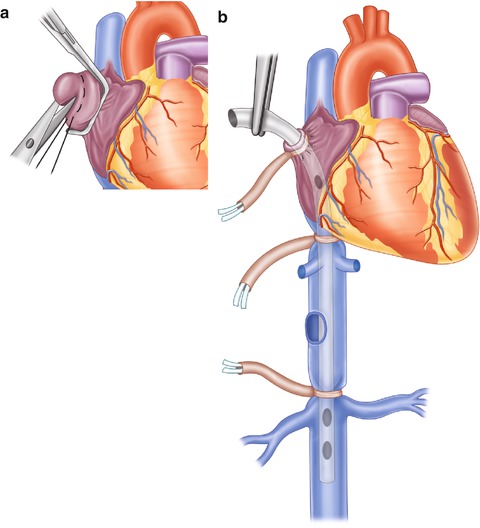

Fig. 9.2
After obtaining the necessary exposure (thoracotomy, mediasternotomy), an opening—along with 2-0 Prolene purse-string suture—is created in the right atrial appendage (a) to provide the access needed for insertion of the atriocaval shunt (b) which is usually a No. 36 chest tube. An extra hole needs to be made at the level of the right atrium. With the chest tube holes being outside of umbilical tape occlusion, blood is directed from the lower half of the body and the kidney through the atriocaval shunt
Although specific ligation of the hepatic artery or portal vein branch supplying a specific portion of the liver is rarely needed, the suspected branch should be isolated, and its occlusion should control hemorrhage while the Pringle maneuver is released. If such is the case, then the identified branch should be ligated.
Liver injury, infrequently, follows the anatomic lines of demarcation delineating the right and left lobes, much less the segments. Anatomic resection, a once popular approach, has poor outcome with high mortality rates. This technique has been, essentially, abandoned. Resectional debridement of devitalized liver is not formal lobectomy but rather a completion of the injury to remove nonviable hepatic tissue and to facilitate vascular control. This usually entails a degree of finger fracture through uninjured liver, which allows visualization of the bleeding raw surface and more direct control. Application of specific liver clamps, such as the Lin clamp, designed to aid in lobectomy, is difficult because of positioning of the injury laceration and maneuvering around the clamp.
Being able to perform a tractotomy of a missile wound should be in a surgeon’s armamentarium when dealing with penetrating hepatic injury. In addition, a variety of methods designed for tamponade of the bleeding missile tract have been described, using various materials. Bluett et al. [8] described a tamponade device with multiple Penrose drains dragged through the liver tract. However, it is more preferable to open the liver and suture or clip-control the bleeding site directly, if possible.
Once the bleeding has been controlled, the large, raw surface of the liver can be problematic, as persistent oozing of bile or blood continues. A viable omental patch sutured to the liver bed is an excellent homeostatic agent and internal drain. Stone and Lamb [9] popularized the omental pack in their initial report. Fabian and Stone [10] reported 90 % successful hemostatic control with this procedure. For large, raw liver surfaces resulting from debridement or tractotomy, the omental patch held in place with several liver sutures is an excellent hemostatic agent. Also, utilization of the argon beam coagulator is another option in addressing bleeding of raw liver surfaces. The argon gas removes the blood from the hepatic tissue and ionizing energy is transmitted. A maximum of 110 ºC is achieved and an eschar is formed.
An alternative to surgical repair of the injured liver is a mesh wrap intended to provide compression, to control bleeding, and to close parenchymal defects. Delaney et al. [11] reported success in six liver injuries controlled in this manner. Brunet et al. [12] reported 35 liver injuries wrapped for control. Sequential CT examination of the patients demonstrated progressive restoration of normal liver architecture.
The premise underlying much of the preceding discussion is that liver bleeding can be controlled. However, even when advanced techniques of liver control are used, hemorrhage control can be precarious, at best. A critical error that can be made when dealing with major liver injury is to continue operative intervention in the face of a hypothermic (less than 32 ºC), acidotic patient who has developed coagulopathy. Although the specific time to make a decision to pack the liver and restore normothermia and coagulation factors is not always clear, the operating surgeon should always have a low threshold to incorporate packing and prepare the patient to be expeditiously transferred to the intensive care unit for aggressive resuscitation and monitoring. Once a patient has required a 10-U transfusion, packing should be seriously considered. Garrison et al. [13], in a review in which they tried to predict the need for packing in severe abdominal injuries, noted that patients with severe injuries, hypothermia, refractory hypertension, coagulopathy, and acidosis need early packing. It needs to be emphasized that large-vessel bleeding must be controlled before packing can be effective.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








