Fig. 1
Genetic map of the transgenic Pod-rtTA/LC1/R26R mice. Pod-rtTA: The podocyte promoter (derived from the human podocin gene, NPHS2) drives expression of the tetracycline-inducible reverse transactivator (rtTA2-M2) in podocytes . LC1: Expression of Cre recombinase and a reporter gene (luciferase, not relevant in this context) are expressed under the control of a “tetracycline-inducible” promoter containing Tet-responsive elements (TRE). Expression is activated reversibly after administration of doxycycline (Dox) in podocytes. R26R: A stop signal (a neocassette) is irreversibly excised by Cre recombination, so that expression of beta-galactosidase (LacZ) is activated irreversibly under the control of the ubiquitously active R26R locus
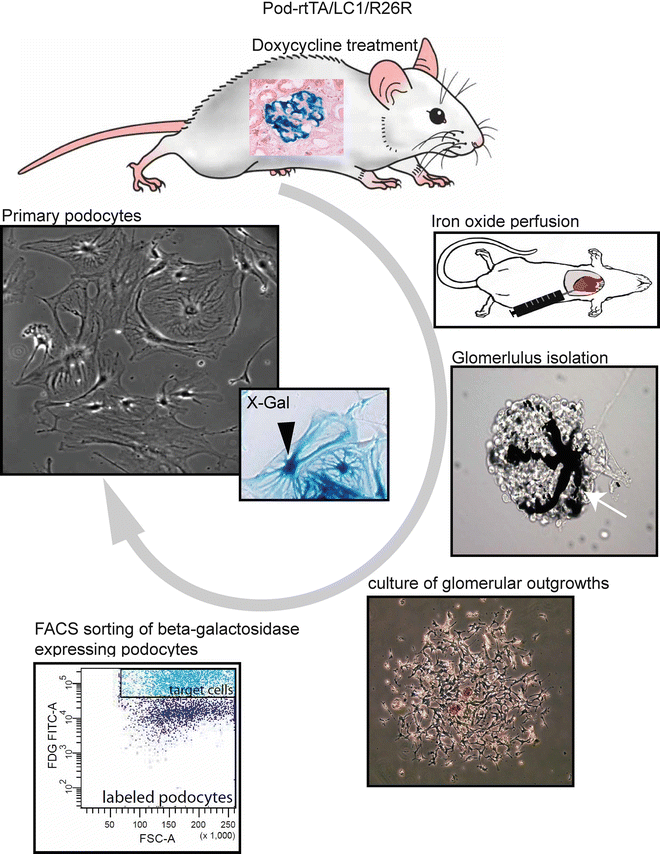
Fig. 2
Flow scheme for the isolation of genetically tagged podocytes . Podocytes are specifically and irreversibly labeled by administration of doxycycline in transgenic Pod-rtTA/LC1/R26R mice. Inset in mouse illustrates X-Gal staining of labeled podocytes (blue staining). The mice are perfused with magnetic iron oxide which accumulates in the capillaries of the glomeruli (white arrow). Glomeruli are isolated and cultured. Single-cell suspensions of the glomerular outgrowths are treated with FDG, which is hydrolyzed by β-gal into an insoluble fluorescent product, and are subjected to FACS. After FACS, X-Gal staining can be performed to assess the purity of the podocyte culture (blue staining, black arrowhead). The primary podocytes form large arborized cell bodies with several intracytoplasmic extensions, i.e., thickenings
In short, podocytes are irreversibly genetically tagged in vivo in the inducible podocyte reporter mouse (Pod-rtTa/LC1/R26R) [6–8]. The glomeruli containing the labeled podocytes, i.e., expressing beta-galactosidase (β-gal), are isolated and cultured. The β-gal-expressing podocytes are directly isolated from the cellular glomerular outgrowths by FACS. The obtained cultures are >97 % pure, based on the β-gal expression.
2 Materials
1.
Pod-rtTA/LC1/R26R mice.
2.
Doxycycline solution: 5 % sucrose (w/v) and 1 mg/mL doxycycline hydrochloride (Fargon GmbH&Co, Barsbüttel, Germany), in normal tap water.
3.
Anesthetic: mixture of 2 % xylazine (20 mg/mL) and 10 % ketamine (100 mg/mL).
4.
Iron oxide solution: normal saline (0.9 % NaCl) containing 0.9 % w/v iron (II,III) oxide (Fe3O4, 98 % purity, 20–30 nm particle powder; Alfa Aesar GmbH, Karlsruhe, Germany).
5.
Collagenase Type 4 (Worthington, Lakewood, NJ, USA).
6.
EGM medium: Endothelial Growth Media (EGM™) Bullet Kit (Lonza, Walkersville, MD USA). To formulate EGM medium, basal medium EBM™ is supplemented with the Bullet Kit components (i.e., human epidermal growth factor [hEGF], hydrocortisone, bovine brain extract [BBE], ascorbic acid, fetal bovine serum [FBS], and gentamicin/amphotericin-B [GA]), according to manufacturer’s protocol. Final concentration of FBS is 20 % v/v.
7.
RPMI medium: RPMI 1640 supplemented with 10 % v/v FBS.
8.
HBSS-Tween: 0.05 % v/v Tween®20 in Hank’s balanced salt solution (HBSS).
9.
Fluorescein di-β-galactopyranoside (FDG) (Molecular Probes, Leiden, the Netherlands).
10.
Cell culture antibiotics: penicillin‐streptomycin solution (10,000 U/mL).
11.
Trypsin‐EDTA cell culture formulation to remove cells from cell culture vessel.
12.
50 mL centrifuge tubes.
13.
Six-well cell culture plates.
14.
75 cm2 cell culture flask.
15.
Automatic FACS cell sorter (e.g., BD FACSAria cell sorter, BD Biosciences, San Jose, CA, USA).
16.
Centrifuge (e.g., Multifuge 3L‐R, Thermo Fischer Scientific Inc., Waltham, MA USA).
17.
Cell strainer, 70 μm (Becton Dickinson, Bradford, MA, USA).
18.
Glutaraldehyde fixative: Prepare a 2 % glutaraldehyde solution in PBS (v/v) from a 25 % glutaraldehyde commercial stock.
19.
Triton™ X-100.
20.
X-Gal staining solution: 1 mg/mL X-Gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS [pH 7.8].
21.




Aqueous mounting media.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







