Fig. 14.1
Anatomy of the extrahepatic biliary tree with typical transverse ultrasonographic planes through the portal pedicle. The right hepatic artery (RHA) usually runs behind the common hepatic duct (CHD), with the origin of the cystic artery (Cystic A) being variable (a). An important variant is an accessory right hepatic artery (aRHA) arising from the superior mesenteric artery present in 15 % of patients (b). The superior mesenteric vein/splenic vein confluence is tear-shaped and often easy to identify (c). LHA left hepatic artery, PHA proper hepatic artery, CHA common hepatic artery, GDA gastroduodenal artery, CBD common bile duct
The cystic artery arises from a variable origin along the length of the proper and right hepatic arteries. Similarly, the insertion of the cystic duct into the common bile duct varies, either running a short course and inserting directly or, more commonly, travelling beside the common hepatic duct for a length prior to insertion. The posterior sectoral duct can also insert low into the common hepatic duct or, rarely, directly into the cystic duct. An uncommon configuration in posterior sectoral duct anatomy is sometimes implicated in the occurrence of bile duct injury at laparoscopic cholecystectomy.
An anatomical variant occurring in 15 % of patients is an accessory or replaced right hepatic artery arising from the superior mesenteric artery (Fig. 14.1b) [4]. Identifying and protecting aberrant vessels is important, particularly during resectional surgery such as extrahepatic bile duct resection and pancreaticoduodenectomy. An accessory left hepatic artery arising from the left gastric artery crosses the lesser omentum in a position out with the portal pedicle. This vessel will not usually be seen on standard sonography of the portal pedicle, unless the probe is moved left and the vessel looked for specifically.
The common hepatic artery is prominent in transverse planes low in the pedicle, appearing greater than its size given the angle of approach (Fig. 14.1c). It can be used to identify the gastroduodenal artery which usually arises at the same level and should not be confused with the right gastric artery (see Fig. 14.4b). The coeliac trunk can usually be visualised dropping behind the pancreas, with the splenic and left gastric artery origins usually visible.
The confluence of the splenic vein and superior mesenteric vein can be identified by its tear-shaped appearance in the transverse plane on ultrasound. Potentially troublesome venous tributaries can often be identified in this area, prior to dissection in pancreaticoduodenectomy. The portal vein can be followed up behind the pancreas towards the liver, a manoeuvre which can often aid the assessment of resectability of malignant disease.
Lymph nodes are present throughout the portal pedicle and may be enlarged as a consequence of disease (see discussion of section “Benign obstruction of the biliary tree”). These are easily identified and measured.
Equipment
A number of different companies now produce versatile high-resolution equipment providing excellent image quality. Real-time B-mode ultrasound images are of course essential, but the ability to add colour flow, power flow and spectral Doppler is now standard. This allows visualisation of blood flow in vessels, which at its simplest can be used in the initial orientation of structures. The technology is now such that even flow in the smallest vessels can be visualised with high sensitivity and resolution. Facilities on the most advanced systems include multi-planar image reconstruction and 3D automated volume measurement.
Various probe options are available including the authors’ preferred T-transducer (Fig. 14.2a) and the I-style finger-grip transducer (Fig. 14.2b). These can be configured with linear or convex arrays, with the former providing a rectangular field of view and the best spatial resolution at the tissue depths typically encountered in hepatobiliary and pancreatic surgery. Laparoscopic probes in the past required to be placed within a sterile plastic sheath containing conductive gel which was awkward and yielded poor image quality. Probes can now be sterilised in ethylene oxide, allowing direct organ contact with improved image in quality. A 12-mm port is required for use and port placement is described below. The tip of the probe is flexible in two planes, allowing most required angles to be achieved through one port (Fig. 14.2c).
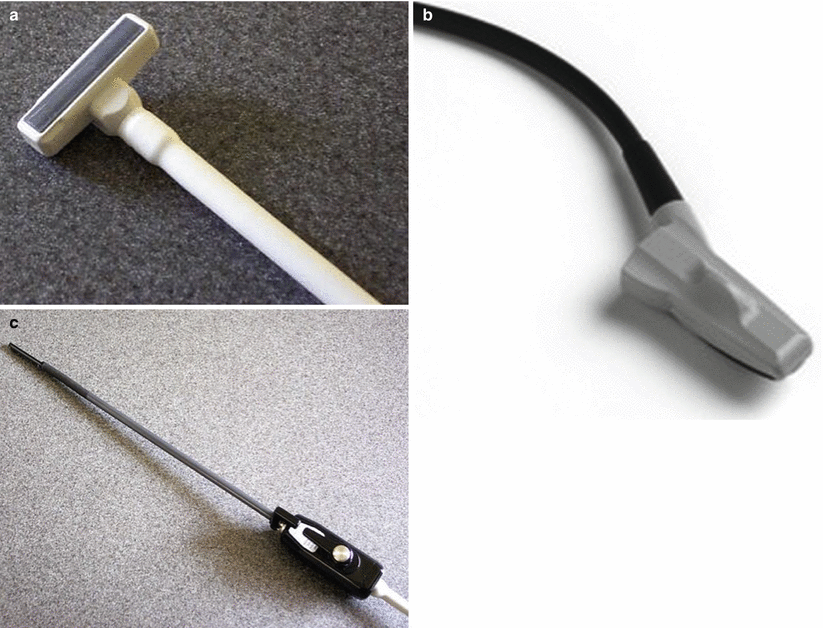

Fig. 14.2
Ultrasound transducers designed for use in surgery: (a) T-style linear probe, (b) I-style convex probe, and (c) laparoscopic probe
Remote control units are available for sterile use and image/video storage can be integrated with institution picture archiving and communication systems (PACS). Specialised probes have been designed that include a needle guide and come with specific software to aid accurate placement for radiofrequency/microwave ablation.
Technique in Open Surgery
The access to the organs of the abdomen afforded by open surgery provides an ideal opportunity for contact ultrasonography. The high-frequency, compact equipment now available can be fully sterilised and placed directly on abdominal viscera to obtain high-resolution real-time images of intra-abdominal structures. This gives the operating surgeon an accurate and flexible tool to assess the anatomy, the nature and the extent of disease, aiding operative decision-making.
Intraoperative ultrasound may be useful in surgery for benign or malignant disease and in both cases the approach is the same. A full visual inspection and manual examination is useful and should be performed initially at laparotomy [5]. Together with the preoperative imaging, this will provide valuable information about the nature and extent of disease. Ultrasonography starts at the hepatoduodenal ligament with examination of the structures of the portal pedicle. Placement of fixed surgical retractors including a specific liver retractor on segment IV may aid access. The use of warm saline around the ligament can help if image quality is poor, but is not always required. It often helps if the operator retracts gently on the duodenum, placing the hepatoduodenal ligament under some tension.
To aid initial orientation, the “Mickey Mouse sign” can be useful (Fig. 14.3). The larger portal vein sitting posteriorly, with the bile duct and hepatic artery in front are reminiscent of the face and ears of the famous cartoon character. “Mickey” can be followed up and down, which acts to keep the probe at right angles to the structures. Care must be taken not to compress structures with the probe. The common bile duct is normally around 8 mm in diameter and can be difficult to visualise when not dilated. It can be differentiated from other portal structures by its hyperechoic wall and absence of flow on Doppler.
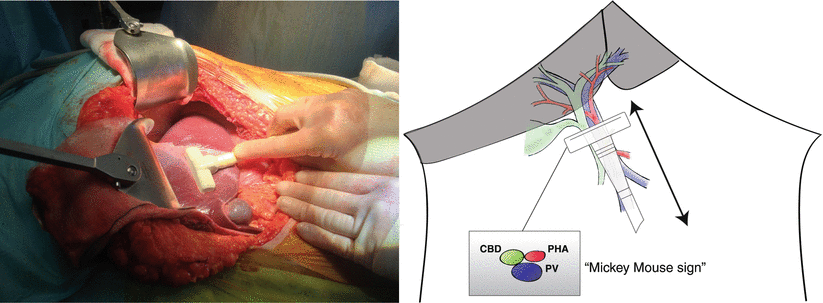

Fig. 14.3
Ultrasonography of the portal pedicle starts with identification of “Mickey Mouse”, formed by the larger portal vein (PV) sitting posteriorly with the common bile duct (CBD) and proper hepatic artery (PHA) in front
Benign Obstruction of the Biliary Tree
Non-malignant obstruction of the biliary tree can result from congenital abnormalities but is more commonly associated with gallstones, benign biliary strictures or a consequence of acute or chronic pancreatitis. In 997 consecutive patients selected for laparoscopic cholecystectomy, operative cholangiography was accomplished in 962 (96 %) [6]. Forty-six patients (4.6 %) had at least one filling defect in the common bile duct, although 12 of these patients had a normal cholangiogram within 48 h (26 % possible false-positive rate) and a further 12 (26 %) had spontaneously passed a stone by 12 weeks. Twenty-two patients (2.2 % of total population) had persistent CBD stones 6 weeks after laparoscopic cholecystectomy.
Figures 14.4 and 14.5 and accompanying videos (Videos 14.1, 14.2 and 14.3, respectively) show typical images obtained with ultrasonography during open surgery. This patient presented with obstructive jaundice and cholangitis on a background of upper abdominal pain. A CT showed dilatation of the extra- and intrahepatic biliary tree with no pancreatic duct dilatation and an obstructing 1.5 cm gallstone in the distal common bile duct. Endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy was performed and a biliary stent placed. The bilirubin remained elevated and the cholangitis persisted and a second ERCP was performed, with placement of a second biliary stent. The stone was impacted and could not be removed at ERCP. Drainage was achieved and the symptoms of sepsis settled. Given the size and position of stone, clearance by open exploration was performed.
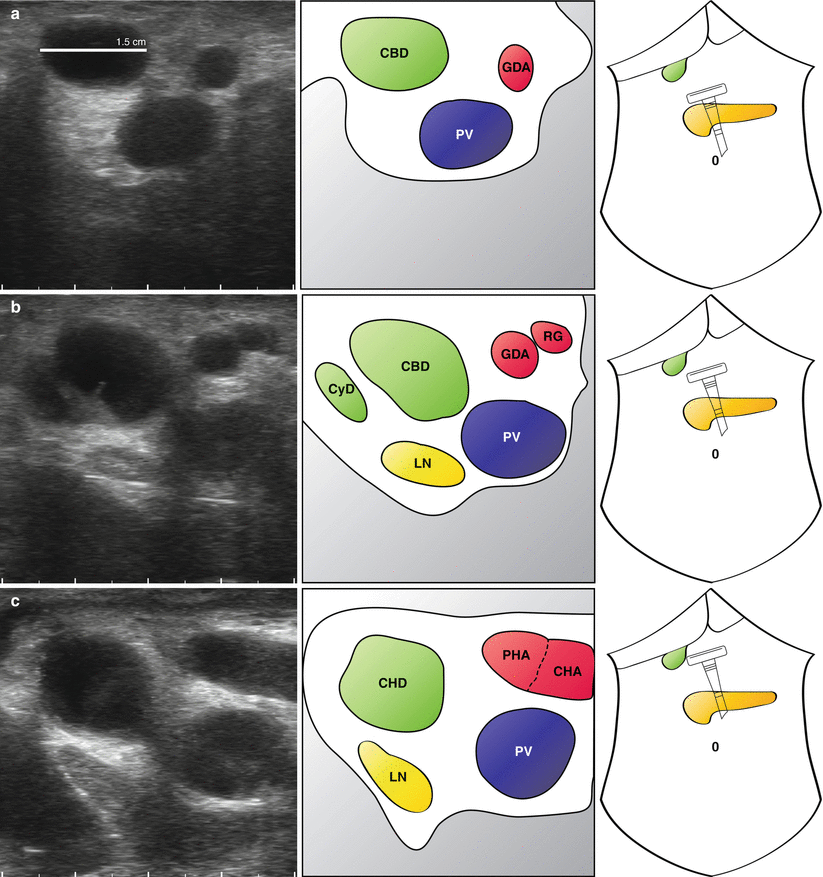
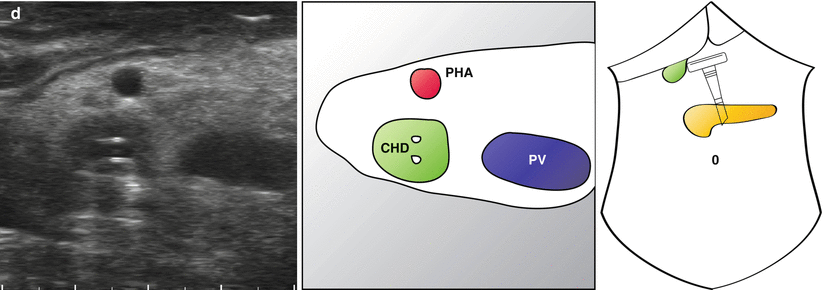
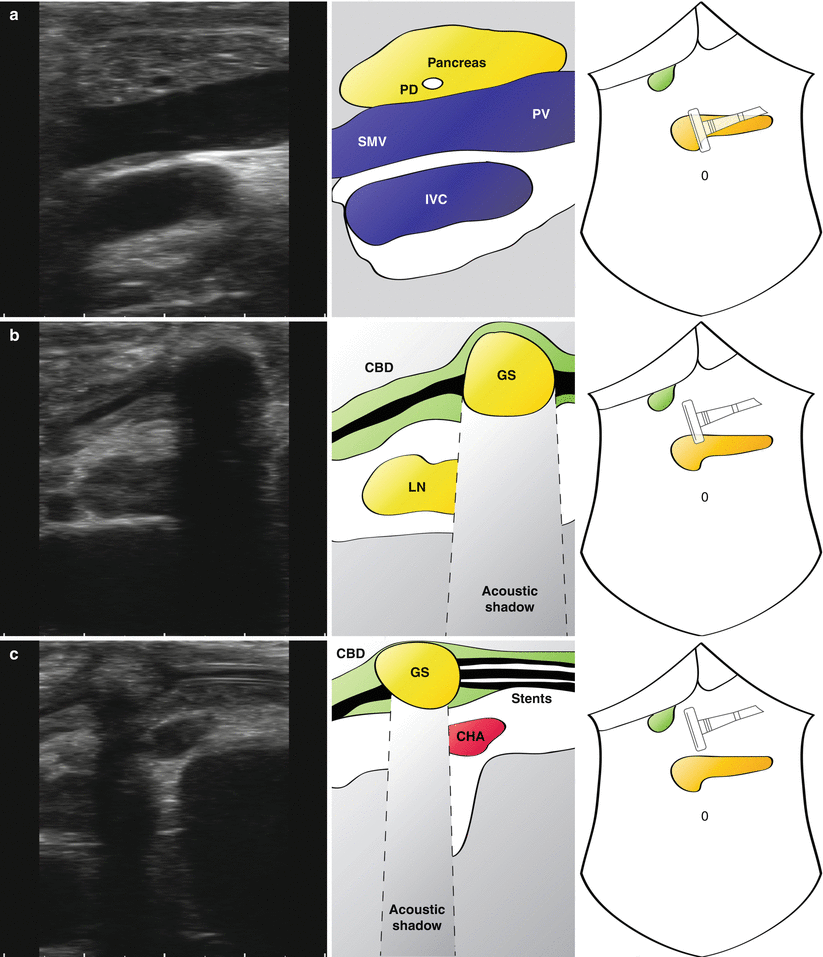
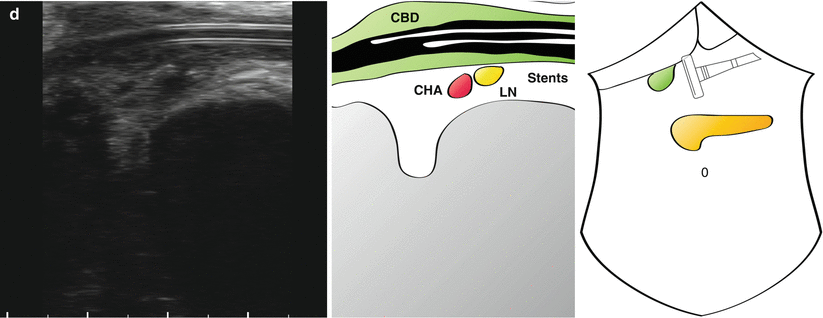


Fig. 14.4
Transverse views of portal pedicle in a patient with biliary obstruction. A full description is provided in the main text. A dilated common bile duct (CBD) is followed up with the portal pedicle (a). The arterial anatomy is clearly seen (a–d) and two plastic stents can be seen within the common hepatic duct (d) (Also see accompanying Video 14.1) GDA gastroduodenal artery, PV portal vein, CHD common hepatic duct, RG right gastric artery, CyD cystic duct, LN lymph node, PHA proper hepatic artery, CHA common hepatic artery


Fig. 14.5
Longitudinal views of portal pedicle in patient with biliary obstruction. The neck of the pancreas can be seen with a visible but non-dilated pancreatic duct (a; PD). A large gallstone (GS) is seen in a thickened common bile duct (b; CBD) (note the prominent acoustic shadow). Stents can be seen in the CBD (c) extending below the level of the stone (d) (Also see accompanying Videos 14.2 and 14.3). SMV superior mesenteric vein, PV portal vein, LN lymph node, CHA common hepatic artery
“Mickey Mouse” is identified and the common bile duct seen to be significantly distended at 1.5 cm (Fig. 14.4a). What initially appears to be the proper hepatic artery is actually a prominent gastroduodenal artery. This is followed up and the origin of the right gastric artery is seen (Fig. 14.4b), just before a large common hepatic artery is visualised (Fig. 14.4c). The cystic duct is long and clearly inserts in a low position (Fig. 14.4b). A lymph node is seen behind the common bile duct which does not look enlarged on ultrasonography and is not suspicious on palpation. Towards the confluence of the left and right hepatic duct, plastic stents are seen within the common hepatic duct, with associated acoustic shadowing (Fig. 14.4d). Some debris can be seen at different levels in the duct.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







