Fig. 4.1
Intestine transplant performed worldwide since 1985
Data from the Scientific Registry of Transplant Recipients (SRTR) show that most of the intestinal transplants have been performed in the US using intestinal graft obtained from cadaver donors (Fig. 4.2) [6]. Differently to what occurred in liver and kidney transplantation , the use of living donors for ITx has been limited mostly because deceased intestinal donors are available. However, optimal deceased intestinal donors are not common either. In fact, despite the fact that the number of patients waiting for ITx is limited, the time spent on the waiting list increased compared to previous years for all candidates on the waiting list. In 2011, 41.7 % of patients listed waited less than 1 year for ITx, while 25.1 % waited between 1 and 2 years, and 33.2 % waited for more than 2 years [6]. Despite the fact that mortality on the waiting list has decreased in the US in recent years compared to the past, it is still reported to be up to 20 % per year on the waiting list [6, 7]. All this suggests that despite the fact that many cadaver donors are available, they are often not utilized because they are not adequate to satisfy the need of patients waiting for ITx.
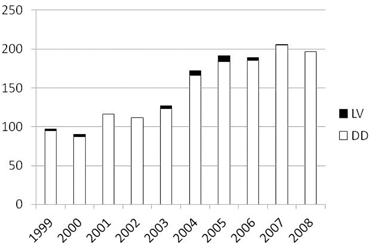
Fig. 4.2
Growth of living donor and deceased donor intestinal transplantation worldwide by era. LV living donors, DD deceased donors
Intestinal Transplant Registry data show that patient and graft survival rates after living donor ITx (LD-ITx) are similar to those obtained with cadaver organs [1, 7–10]. However, it is important considering that most of the LD-ITx surgeries were performed in low-volume centers (often as isolated cases in low-experience centers), while the best results obtained with ITx from cadaver donors are obtained in high-volume centers, suggesting that increasing the use of LD-ITx could further improve outcome. In fact, the use of intestinal grafts from living donors, when compared to cadaver donors, offers important advantages with low risks, and these are summarized in Table 4.1.
Table 4.1
Potential advantages and disadvantages using intestinal grafts from living compared to deceased donors
Advantages |
Eliminates waiting time in cadaver donor list |
Can be used in countries where TPN and deceased donors are not available |
Use of hemodynamically stable donors |
Elective case, allows better donor bowel preparation and optimization of recipient clinical condition |
Short cold ischemia time |
Optimal donor–recipient HLA matching |
Small graft can be accommodated in retracted abdominal cavity |
Disadvantages |
Risk for the donor |
Short graft and smaller vascular pedicle |
As mentioned above, LD-ITx virtually eliminates waiting time and could further decrease morbidity and mortality on the waiting list compared to the activity seen on the deceased donor list. LD-ITx also would allow transplantation in developing countries where TPN and deceased donor are not easily available. Furthermore, because LD-ITx is an elective procedure, it can be performed when the donor and the recipient conditions are optimal, and the donor bowel preparation can be easily performed, leading to a decreased risk of infectious complications. In an analysis of 50 pediatric ITx recipients, it has been shown that the length of graft preservation was the most significant factor in inducing bacterial translocation [11, 12]. This phenomenon can contribute to the high rate of infections seen during the early posttransplant period also considering that this coincides with the timing of maximum amount of immunosuppression given to the patient.
The Achilles’ heel of bowel transplantation remains its extreme sensitivity to preservation injury. Deceased donors are often subject to either cardiac arrest or resuscitation, to prolonged periods of hypotension, or use of vasopressors. All these could result in splanchnic hypoperfusion, which can trigger ischemia/reperfusion injury even before the intestine is procured from the donor. Cold preservation of the graft can be also extended due to the distance between procurement and transplant centers. This can further increase such injury considering that specific preservation solutions designed for intestinal grafts are not yet available. The use of living donors can obviate these problems, since the donor is a healthy individual, hemodynamically stable, and with consequent normal intestinal perfusion. Furthermore, the short cold ischemic time prior to revascularization also improves graft quality, virtually eliminating ischemia/reperfusion injury, and may reduce the rate of posttransplant infections [12].
Another not negligible benefit of LD-ITx is immunologic [8, 9]. A living donor is often a relative of the recipient due to the strong emotional involvement that justifies the donation. Living related donors have a closer distribution of human leukocyte antigens (HLAs) with the recipient that could contribute to an immunologic advantage. This is supported by the experience of HLA-matched transplants performed between homozygote twins [13]. This point might be challenged since in recent times, a significant decreased rate of rejection has been also observed using HLA-unmatched deceased donors [1, 14]. However, this improvement has been accomplished using more potent immunosuppression and induction therapy with polyclonal antibodies. However, a similarly low rate of rejection is seen using living related HLA-matched donors using a less potent immunosuppressive regimen [8, 9, 15]. An additional benefit can be offered performing LD-ITx at an earlier stage of the disease, since long-term TPN and indwelling venous catheters can be associated with priming of the immune system, as recently suggested [16–18].
Naturally, potential disadvantages are also associated with the use of living donors. The main disadvantage remains the risk for the donor, which includes early surgical complications of bowel resection as well as potential long-term impairment of intestinal absorption. The procedure-specific risks for the live intestinal donor are given in Table 4.2. Specific data on living intestinal donors are limited at this time, and no serious complications have been reported. The potential risk can be hypothetically calculated, using a parallelism with general surgery-related data of small bowel resection; about 3–5 % of the donors could develop a small bowel obstruction [19–24]. In large series, the mortality rate for patients with small bowel obstruction is approximately 2 % for the lifetime of the patient [25]. A brief and self-limited period of diarrhea has been reported after intestinal donation for LD-ITx [15]. Although weight loss and dysvitaminosis are not reported, they represent potential risks of this procedure.
Table 4.2
Potential procedure-specific risk for the live intestinal donor
Short bowel syndrome |
Small bowel obstruction |
3–8 % |
3 % mortality |
Dysvitaminosis |
Weight loss |
Diarrhea |
Another disadvantage is associated with the increased risk of vascular thrombosis, related to the smaller vascular pedicle used compared to grafts obtained from cadaver donors. Nevertheless, these risks can be reduced with a careful and appropriate surgical technique [26, 27].
Donor Selection and Evaluation
General Considerations
The potential donor should be an individual in good health with no history of previous intestinal or abdominal surgery, and with no underlying chronic medical illnesses that would increase the surgical operative risk.
Once a potential donor is identified, the initial step should consist of a meeting with the surgeon to describe the procedure, as well as risks and benefits and the steps involved in the workup. During this initial visit, the potential donor can be screened with an ABO blood type determination. If this is compatible and the candidate is willing to continue the workup, an HLA test and histocompatibility testing by T-cell crossmatch should be performed. Crossmatch should be negative, and among multiple donor candidates, the one with the best HLA match should be preferred and should be directed to continue the workup. The cornerstone of success is the identical or compatible HLA. For this reason, it is preferred that living donors be relatives of the recipients. The donor can also be unrelated to the recipient but should have a compatible HLA and close emotional relationship. This condition and the absence of any financial interest or coercion for donation are of paramount importance in LD-ITx, just like any other type of live organ donation. The screening process should exclude active or uncontrolled psychiatric disorders, and ensure the altruistic nature of the donation. The institution’s ethical committee should separately evaluate the donor to ensure that there is full understanding of the limited information regarding the short- and long-term risks associated with intestinal donation.
Full Evaluation
Once the potential donor has completed these initial steps, a series of tests are mandatory for the live donor evaluation (Table 4.3). Based on the available clinical experience with LD-ITx, a limit of 60 years of age is advisable. The minimal age is only determined by legal ability to consent to the procedure. High body mass index (> 30) may not affect graft quality and does not constitute, per se, an absolute contraindication to live donation, though general surgical experience indicates that a high body mass index (> 30 kg/m2) may increase the risk of surgical complications after intestinal resection.
Table 4.3
Donor and recipient preoperative workup
Workup | Donor | Recipient |
|---|---|---|
Laboratory tests | Blood group system (ABO) and HLA | Blood group system (ABO) and HLA |
CBC with differential | CBC with differential | |
Coagulation panel (PT/INR, PTT) | Coagulation panel (PT/INR, PTT) | |
Liver chemistries, amylase, lipase | Liver chemistries, amylase, lipase | |
Renal chemistries and electrolytes | Renal chemistries and electrolytes | |
Basic metabolic panel | Basic metabolic panel | |
Urinalysis and culture | Urinalysis and culture | |
Stool culture | Stool culture | |
Vitamin A, D, E, K, and B12 | Vitamin A, D, l, K, and B12 | |
Ammonia, alpha fetoprotein, lipid profile | Ammonia, alpha fetoprotein, lipid profile | |
Baseline serum citrullin level Hypercoagulable workup (i.e., protein C, protein S, antithrombin III, factor V Leiden mutation) when indicated | ||
Serology | Hepatitis screen, HIV, CMV IgM and IgG, EBV IgM and IgG, ZVZ IgA EIA, | Hepatitis screen, HIV, CMV IgM and IgG, EBV IgM and IgG, ZVZ IgA EIA, syphilis |
For pediatric patients also: lgG and IgM titers for herpes, varicella, mumps, measles, and rubella | ||
Cardiac assessment | Chest X-ray | Chest X-ray |
EKG (12 lead) and electrocardiography | EKG (12 lead) and electrocardiography | |
GI assessment | D-xylose and fecal fat absorption studies Screen for celiac sprue | D-xylose and fecal fat absorption studies |
Imaging studies | CT abdomen with intravenous contrast | CT abdomen with intravenous contrast |
3D angio CT scan or SMA angiogram | Barium enema | |
Upper GI with small bowel follow through Gastric emptying study | ||
Venogram | ||
Other | Formal psychosocial assessment | Liver biopsy |
Ethics committee evaluation | Colonoscopy |
A comprehensive metabolic panel should be obtained. Blood test results that confirm donor infection with HIV, HCV, or HBV are contraindications for living intestine donation.
Specific considerations must be used for genetically related donors of potential recipients who have a genetic or familial intestinal disease. Despite the fact that no data are available at this time, it is possible that the related donor might develop the same condition later in life. At the present time, it is advisable not to consider these donors and eventually to screen them to rule out the same genetic disorder.
Imaging studies are performed to rule out underlying or occult pathology and to specifically delineate the intestinal vascular anatomy. A computed tomography (CT) or magnetic resonance (MR) angiography is performed, possibly with computerized three-dimensional (3D) reconstruction, if available. In case, these techniques are not available or are inadequate, a traditional angiogram can be also performed. Angiography is performed to evaluate the superior mesenteric artery (SMA) anatomy, to ensure a normal vascular distribution to the small bowel with particular attention to the right colic and ileocolic arteries and the terminal branches of the SMA, and to exclude the presence of atherosclerotic disease and abnormal anatomy. If more than one donor is available, patients with a single distal arterial pedicle should be preferred to patients with multiple vessels. These vessels usually originate caudal to the takeoff of the right colic artery and must be spared during procurement to provide adequate blood supply to the cecum, terminal ileum, and ileocecal valve (Fig. 4.3).
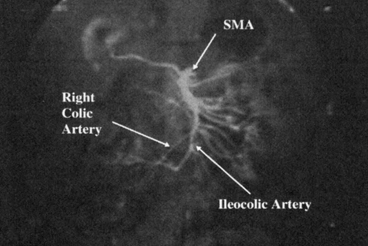
Fig. 4.3
Mesenteric angiography
Indications and Recipient Selection
The indications for ITx are identical using either a living or a deceased donor. This transplant should be considered for patients with irreversible intestinal failure requiring TPN. This can be caused by short gut syndrome (Figs. 4.4 and 4.5) related to the loss of over 70 % of the native small bowel length (< 100 cm of residual intestine), defective gastrointestinal (GI) motility, impaired enterocyte absorptive capacity, genetic malformations of the GI tract or abdominal wall, or neoplastic disease [1]. The irreversibility of intestinal failure is based on the length and function of the remaining native bowel and its inability to provide sufficient fluid and nutritional support. Intestinal rehabilitation can correct this condition in up to 50 % of patients requiring chronic TPN, and should be always attempted before considering transplantation [28, 29].
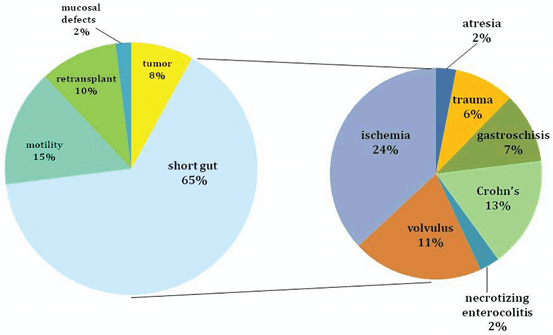
Fig. 4.4
Indications for intestinal transplantation in adults
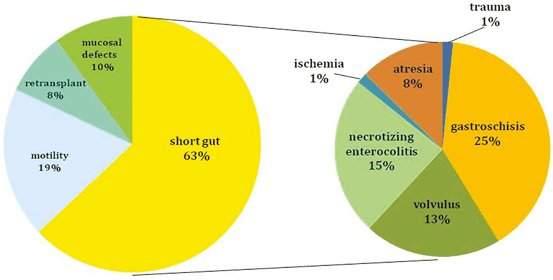
Fig. 4.5
Indications for intestinal transplantation in children
Inclusion Criteria
A patient diagnosed with intestinal failure is not automatically considered an ITx candidate. Usually, these patients are considered transplant candidates only when TPN-related complications arise. These criteria are summarized in Table 4.4.
Table 4.4
Potential procedure-specific risk for the live intestinal donor
Failure of parenteral nutrition, as defined by the Centers for Medicare and Medicaid Services |
Impending or overt liver failure due to TPN-induced liver injury |
Thrombosis of two or more central veins |
Two or more episodes per year of catheter-related systemic sepsis that requires hospitalization |
A single episode of line-related fungemia, septic shock, or acute respiratory distress syndrome |
Frequent episodes of severe dehydration despite intravenous fluid supplementation in addition to TPN |
Impending Liver Failure Due to TPN-Induced Cholestasis
TPN-induced cholestasis is a condition of impaired canalicular secretion of bile, characterized by bile duct regeneration, portal inflammation, and fibrosis. It is diagnosed in patients receiving TPN who develop cholestasis not due to other liver diseases or biliary obstruction. The clinical manifestations include elevated serum bilirubin and/or liver enzymes, splenomegaly, thrombocytopenia, gastroesophageal varices, coagulopathy, stomal bleeding, or hepatic fibrosis/cirrhosis. Its progression could be very rapid, and in some patients, liver cirrhosis may develop in a few months [30].
Vascular Access
This can be consequent to the thrombosis of the major central venous system, such as jugular, subclavian, and femoral veins. Thrombosis of two or more of these vessels is considered a life-threatening complication and a failure of TPN therapy. The sequelae of central venous thrombosis are the lack of access for TPN infusion, fatal sepsis secondary to infected thrombi, pulmonary embolism, superior vena cava syndrome, or chronic venous insufficiency.
Frequent Line Infections and Sepsis
The development of two or more episodes per year of systemic sepsis, secondary to line infection that requires hospitalization, also indicates failure of TPN therapy. A single episode of line-related fungemia, septic shock, and/or acute respiratory disease syndrome (ARDS) is considered TPN failure.
Frequent Episodes of Severe Dehydration Despite Intravenous Fluid Supplement in Addition to TPN
Under certain medical conditions, such as secretory diarrhea, in GI tract that cannot be reconstructed, the loss of the GI and pancreatobiliary secretions exceed the maximum intravenous infusion rates that can be tolerated by the cardiopulmonary system.
Exclusion Criteria
Several conditions also preclude intestinal transplant. The exclusion criteria are summarized in Table 4.5.
Table 4.5
Contraindications for live donor intestinal transplant
Relative contraindications |
Age less than 6 months or greater than 70 years |
Human immunodeficiency virus (HIV) seropositive |
Active substance abuse |
Absolute contraindications |
Significant uncorrectable cardiopulmonary insufficiency |
Incurable malignancy |
Active systemic infections |
Severe systemic autoimmune disease |
Acquired immune deficiency syndrome |
Preoperative Workup
Once a patient with intestinal failure is considered for a transplant, a specific evaluation is performed. The purpose of the evaluation is to determine if the patient would benefit from ITx; to rule out contraindications; and to improve, if possible, the current medical management of these patients. The workup performed in the recipient is similar for either a deceased donor or a live donor (see Table 4.3). The workup is performed by a multidisciplinary team consisting of a transplant surgeon, gastroenterologist, nutrition specialist, cardiologist, anesthesiologist, infectious disease specialist, psychiatrist, and social worker. A multidisciplinary committee discussion and presentation of each case are advisable.
Radiographic Imaging Studies
It is imperative to know the anatomy of the recipient pretransplant. If numerous intestinal resections have been performed over a long period of time, often previous medical records are not available or are inaccurate in recording the remaining portion of the intestine and its length after each surgery. In addition, intraoperative evaluation of the anatomy is often difficult due to the scarring and adhesions found. Upper and lower GI series with contrast will allow one to visualize the portion of residual gut, its position in the abdominal cavity, and its length. It is not uncommon that during a workup, a longer-than-expected segment of small or large intestine is identified, and this might allow different strategies than transplantation, such as surgical recanalization of the residual intestine, intestinal rehabilitation, or other surgical elongation procedures. Abdominal CT scan (or MR imaging) with contrast is also used to rule out malignancies or other undiagnosed diseases.
Patency of the upper and lower body veins must be established by venogram, since duplex scan might not be sufficiently sensitive for this purpose. Thrombosis of these vessels is not uncommon in patients receiving TPN. Thrombosis can cause inability to cannulate the vessels, and can cause superior vena cava syndrome when the inferior vena cava (IVC) is clamped. The patient may have patency only of the femoral veins. Complete lack of venous access could be a contraindication for ITx.
Electrocardiography and echocardiogram are used to determine the cardiac function and any valvular lesions, and should be accompanied by a cardiologic evaluation and clearance for surgery. Stress test or cardiac catheterization may also be performed, if indicated.
Additional Diagnostic Procedures
Liver biopsy may be indicated in patients with intestinal failure and hepatic dysfunction. TPN-induced cholestatic liver injury can be reversed by isolated intestinal transplant or by restoring intestinal integrity [31]. However, in the presence of liver cirrhosis or portal hypertension, a patient with intestinal failure requires a combined liver–ITx. This can be performed using a deceased donor. However, as an option, liver transplant from a deceased donor can be followed by intestinal transplant from a living donor, if an intestinal graft is not attainable from the same donor at the same time. In addition, in pediatric patients, combined liver–ITx from a living donor has been recently reported [10].
Further assessment of associated liver disease (portal hypertension, coagulopathy, ascites, hyperdynamic circulation, hepatopulmonary syndrome, and hepatic encephalopathy) should be done, if indicated.
Patients with familial polyposis should be evaluated for the presence of polyps in the remaining portion of the Gl tract. Patients with dysmotility disorders may require an assessment of the stomach to evaluate functional abnormalities. Children with pseudo-obstruction may require urologic assessment because as many as a third may have a dysfunctional urinary tract. Children with necrotizing enterocolitis may require a full neurologic and pulmonary workup to exclude the possibility of associated intraventricular hemorrhage and bronchopulmonary dysplasia.
Surgical Technique
Background
The transplantation of an intestinal graft from a live donor, by definition, involves the transplantation of a segment of the small intestine. Central caveat of the donor operation is to provide adequate length of intestine to the recipient to ensure enteral autonomy, while preserving enough small bowel length in the donor. The appropriate length and the anatomic origin of the segmental graft to harvest is the cornerstone of a successful transplant.
Anatomical Considerations
The arterial blood supply to the small intestine is from the superior mesenteric artery (SMA) . The basic pattern of distribution of the intestinal arteries generally includes 5 arteries arising on the left of the SMA above the origin of the ileocolic artery and 11 below that level. Eight additional arteries usually originate from the ileal branch of the ileocolic artery [32]. These intestinal vessels branch a few centimeters from the border of the intestine to form arterial arcades connecting the intestinal arteries with one another. Proximally, a single set of arcades is present; distally, there are usually several sets of arcades. These arches form the primary interconnections of the arterial supply. From arches and arcades, the vasa recta arise and pass without cross-communication to enter the intestinal wall. A complete channel may also exist from the posteroinferior pancreaticoduodenal artery that is parallel to the intestine and joins the marginal artery of Drummond of the colon. The terminal ileum, ileocecal valve, and right colon receive blood supply also from the right colic artery and ileocolic artery, often sharing a common origin, connected by the marginal artery of Drummond to the terminal branches of the SMA (Fig. 4.6). In 5 % of the population, blood supply to these structures is guaranteed only by the ileocolic artery, as the marginal artery is incomplete (Fig. 4.7). The venous drainage of the small intestine is less complex than the arterial vessels, merging in the jejunal and ileal veins, and into the superior mesenteric vein and portal vein.
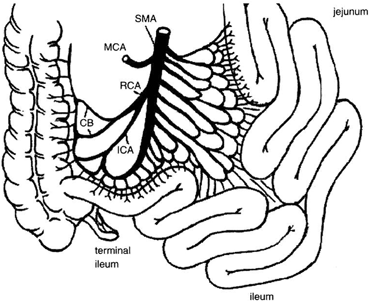
Fig. 4.6
Vascular supply to the small intestine from SMA. The terminal ileum, ileocecal valve, and right colon receive blood supply also from the right colic and ileocolic arteries, and are connected by the marginal artery of Drummond. CB colic branches, ICA ileocolic artery, MCA middle colic artery, RCA right colic artery, SMA superior mesenteric artery. (From Tan HP, Marcos A, Shapiro R, Living donor organ transplantation, 1st edition, copyright © 2007, Informa Healthcare. Reproduced with permission of Informa)
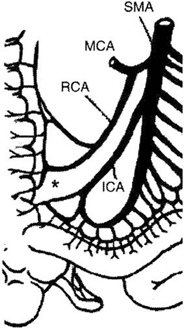
Fig. 4.7
Vascular supply to the small intestine from SMA. The marginal artery is incomplete in 5 % of the population. IMA incomplete marginal artery, ICA ileocolic artery, MCA middle colic artery, RCA right colic artery, SMA superior mesenteric artery. (From Tan HP, Marcos A, Shapiro R, Living donor organ transplantation, 1st edition, copyright © 2007, Informa Healthcare. Reproduced with permission of Informa)
From a technical standpoint, the ileum offers the advantage of a larger vascular pedicle if the distal portion of the SMA is used. This vessel can be transected below the takeoff of the right colic artery to avoid hypoperfusion of the terminal ileum, and the preservation of the ileocecal valve in the donor (Fig. 4.8). At this level, this artery is commonly single, but could also consist of two or more branches. Additionally, despite the fact that both jejunum and ileum have the ability to adapt following intestinal resection, the ileum has the advantage of allowing structural and functional adaptation [33, 34].
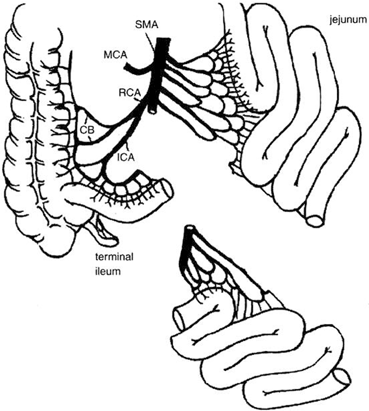
Fig. 4.8
From a technical standpoint, the ileum offers the advantage of a larger pedicle if the distal portion of the superior mesenteric artery is used. This vessel can be transected below the takeoff of the right colic artery to avoid hypoperfusion of the terminal ileum and ileocecal valve, which are always preserved in the donor. CB colic branches, ICA ileocolic artery, MCA middle colic artery, RCA right colic artery, SMA superior mesenteric artery. (From Tan HP, Marcos A, Shapiro R, Living donor organ transplantation, 1st edition, copyright © 2007, Informa Healthcare. Reproduced with permission of Informa)
Despite these advantages, the jejunum has been also used in early experience for the possible immunologic advantages, since it has been reported, from small-animal studies, that acute rejection is less severe in the jejunum [35, 36]. However, the vascular distribution to the jejunum makes the operation more complex, as the segmental graft obtained has numerous arterial branches needing multiple anastomoses in the recipient to obtain adequate revascularization for the graft (Fig. 4.9). Jaffe et al. reported attempts at proximal small bowel transplantation involving complex vascular reconstruction that resulted in vascular complications [35]. In addition, the early clinical experience from the same group did not show a clear immunologic advantage with the use of jejunal segmental grafts.
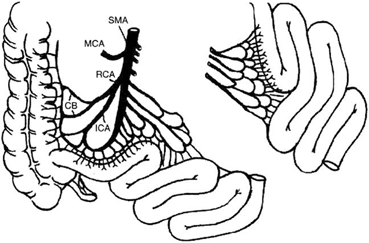
Fig. 4.9
The vascular distribution to the jejunum makes the operation more complex as the segmental graft obtained has numerous arterial branches needing multiple anastomoses in the recipient to obtain adequate revascularization of the graft. CB colic branches, ICA ileocolic artery, MCA middle colic artery, RCA right colic artery, SMA, superior mesenteric artery. (From Tan HP, Marcos A, Shapiro R, Living donor organ transplantation, 1st edition, copyright © 2007, Informa Healthcare. Reproduced with permission of Informa)
Optimal Length of the Segmental Graft
The appropriate length of the human alimentary tract to be resected has proven to be surprisingly difficult to measure. The length of the small intestine in deceased donors was reported to be from 10 to 40 feet, with an average of 20.5 feet or 624.8 cm [37]. In vivo measurements using an intraluminal method provided an average of 8.5 feet or 258 cm [38]. This discrepancy is attributed to the postmortem loss of longitudinal muscle tune of the small intestine that can lead to an increase in length, up to 135 % in a few hours, as shown in animal studies [39]. For the purpose of ITx, intestinal measurements are performed in a live subject, but being under general anesthesia, the effect of the pharmacologic agents used might affect the intestinal distension, motility, and length [40–43].
For these reasons, the calculation of a generic optimal length of small bowel graft to resect can be difficult. In each individual case, the entire small bowel should be measured from the ligament of Treitz to the ileocecal valve, using a sterile surgical tape. Once the length of the entire small bowel is determined in a particular patient under a specific anesthetic agent, a final determination of the segment to be removed is made. Although it is relatively easy to determine when an intestinal segment is too short, the long-term impact to the donor of the resection of a longer segment is unknown. Deltz reported a transplant of a 60-cm segment of distal jejunum and proximal ileum, whereas, Morris used a similarly long segment of distal ileum, ileocecal valve, and a portion of the cecum [44, 45]. Despite these early successes, a length of 60-cm small bowel has been generally inadequate to provide a TPN-free condition [44], and resection of the ileum, ileocecal valve, and cecum can have a negative impact on the function of the remaining donor bowel (e.g., increased transit time and vitamin B12 deficiency) [45].
From the short-bowel syndrome literature, a segment of approximately 1 m has been reported to be sufficient to ensure adequate absorption [29]. However, this depends on the presence of the ileocecal valve or part of the colon. Considerations in the recipient anatomy also play an important role in determining the length of the intestinal segment to remove in the donor. A recipient with no colon or ileocecal valve, for example, will require a longer segment compared with a patient where these structures are present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





