As men age, there is an increase in the frequency of pathologic diseases affecting the genitourinary tract. Most notable among these changes are the rising prevalence of lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) and erectile dysfunction (ED). The pathogenesis of these conditions seems to be multifactorial and includes age-related changes in the nervous system and neuroregulatory factors, such as nitric oxide and RhoA/Rho-kinase. Various pharmacologic agents that target these pathways, such as α-blockers and PDE-5is, underscore the contribution of neuroregulatory factors on the development of LUTS/BPH and ED.
As men age, there is an associated increase in the frequency of pathologic diseases affecting the genitourinary tract. Most notable among these changes are the rising prevalence of lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) and erectile dysfunction (ED). The pathogenesis of these conditions seems to be multifactorial and includes age-related changes in the nervous system and neuroregulatory factors, such as nitric oxide (NO) and RhoA/Rho-kinase. Although some of these neuromodulatory effects are directly associated with the aging process, many are secondary to comorbid conditions related to aging, such as the metabolic syndrome (MSx), diabetes, and hypogonadism. The success of several widely used pharmacologic interventions reflect the importance of neuronal influences on urologic disease in aging men.
Normal innervation of the bladder, prostate, and penis
The male lower urinary tract is innervated by both the somatic and the autonomic nervous systems (ANS). The autonomic component consists of pelvic parasympathetic and lumbar sympathetic nerves. In addition, the role of other neuroregulatory pathways, such as NO and RhoA/Rho-kinase, are increasing being described in the lower urinary tract. These nerves serve important roles in the regulation of urine storage, micturition, erectile function, and ejaculation.
Bladder
Parasympathetic nerves help to control micturition through innervation of bladder detrusor muscles ( Fig. 1 ). This interaction is mediated by acetylcholine (ACh) binding to detrusor muscarinic receptors. There are 5 subtypes of muscarinic receptors. Although there is more expression of the M 2 subtype in the bladder, the M 3 subtype is the most important for initiating detrusor contractions. Muscarinic receptor binding leads to a cascade of signaling events that results in the activation of phospholipase C (PLC) and the hydrolysis of inositol 1,4,5-trisphosphate (IP 3 ). Subsequently, IP 3 causes an increase in intracellular calcium, which binds calmodulin (CaM) to activate myosin light chain kinase (MLCK). Phosphorylation of myosin regulatory light chain (MLC) by MLCK causes MLC to activate myosin ATPase and enhance muscle contractility. MLC is dephosphorylated by MLC phosphatase (MLCP), which results in muscle relaxation. In addition, smooth muscle cell (SMC) contraction is also regulated through the RhoA/Rho-kinase pathway, also called the alternate pathway. Activation of Rho-kinase occurs through the mediator RhoA, a GTPase. RhoA is regulated by various factors, including activation through muscarinic receptors. Rho-kinase inhibits MLCP by phosphorylation, permitting continued activity of MLC in muscle contraction, which indicates that the RhoA/Rho-kinase pathway allows for MLC activation at lower levels of calcium by inhibiting MLCP, known as calcium sensitivity.
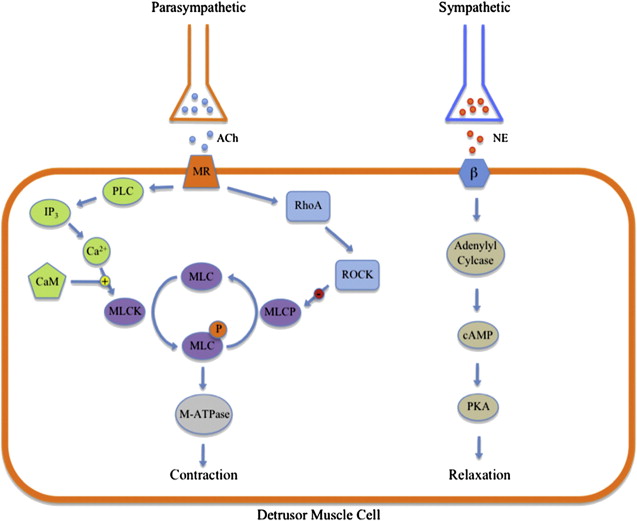
Bladder contraction and relaxation is also controlled by sympathetic innervation (see Fig. 1 ). There are 9 adrenergic receptor subtypes: 3 α 1 , 3 α 2 , and 3 β receptor subtypes. Stimulation of β-adrenergic receptors by norepinephrine leads to detrusor muscle relaxation. The β 3 receptor subtype is the most abundantly expressed and seems to be the most important subtype for this interaction. Signaling through the β receptor activates adenylyl cyclase to increase the levels cyclic AMP (cAMP). Protein kinase A (PKA) is activated by cAMP, causing smooth muscle relaxation. In contrast, bladder outlet contraction is mainly under the control of α-adrenergic receptor stimulation by norepinephrine. This occurs mainly through the α 1A subtype. Activation of this receptor leads to G protein hydrolysis of IP 3 . The resulting increase in intracellular calcium increases muscle tone in the bladder neck, urethra, and prostate.
In animal models, NO has been shown to have a role in the regulation of smooth muscle tone in the bladder neck. Recent evidence also indicates that NO may play a role in SMC relaxation in the human bladder neck. NO is produced by NO synthases (NOS), of which 3 isoforms are known: neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS). The presence of NO leads to the production of cyclic guanosine monophosphate (cGMP) in SMCs. cGMP subsequently activates protein kinase G (PKG), initiating a phosphorylation cascade that ultimately leads to SMC relaxation.
Prostate
The prostate is innervated by both the parasympathetic and sympathetic components of the ANS. Parasympathetic innervation helps to regulate the function of prostate epithelial cells through the activity of acetylcholine (ACh) on muscarinic receptors. It seems that the M 1 subtype is most commonly associated with these epithelial cells. There are also some data to suggest that a significantly smaller number of M 2 subtypes are found on stromal cells. Animal models indicate that parasympathetic stimulation increases prostatic secretions mainly through activation of these epithelial M 1 receptors.
Sympathetic innervation of the prostate is thought to be integral to the regulation of stromal cellular elements and may provide prostatic muscle tone. α-Adrenergic receptors are concentrated in the stromal cells and in prostatic blood vessels. Stromal cells express α 1 receptors, more specifically α 1A . Given the sympathetic innervation to the stroma, sympathetic nerves play an important part in regulating prostatic SMC tone and help to coordinate prostatic secretion of fluid. Prostatic SMC tone also seems to be regulated by the RhoA/Rho-kinase pathway. RhoA and Rho-kinase have been shown in human prostate tissue. Inhibition of Rho-kinase in prostatic tissue by the inhibitor Y-27632 decreased norepinephrine-induced contraction at constant calcium concentration.
Prostate growth is also influenced by autonomic neural input. This relationship has been well documented in various animal models. For example, McVary and colleagues showed a positive association between the autonomic tone of the prostate and the rate of growth of the gland. Removal of this innervation is associated with a regression in prostate gland weight, DNA content, and protein content. These results support the notion that increased sympathetic input contributes to increased prostate growth and bladder outlet obstruction.
Nonadrenergic, noncholinergic neurotransmitters such as NO are also present in the prostate and seem to influence normal prostatic SMC tone and growth. nNOS and eNOS have been identified in the prostate by various methods. It seems that eNOS is localized to endothelial cells of the prostatic vessels. In comparison, nNOS is concentrated around nerve terminals supplying stromal SMCs and glandular structures.
Penis
The penis receives somatic, parasympathetic, and sympathetic efferent innervation. Somatic neural input to the bulbocavernosus and ischiocavernosus muscles improves penile rigidity and assists in expulsion of ejaculate. Achieving tumescence involves interactions between the parasympathetic nerves and the vascular endothelial cells within the corpora cavernosa. NO is the mediator of this interaction. Cavernosal nerves express the neuromodulator nNOS. NO is also produced by the local endothelial cells via eNOS. NO from both sources triggers the production of cGMP by cavernosal SMCs, ultimately leading to SMC relaxation and subsequent penile erection. The enzyme phosphodiesterase-5 (PDE-5) is responsible for cGMP degradation, which leads to detumescence. In addition, sympathetic neural input provides inhibition to parasympathetic neurons and also contributes to the relaxation of cavernosal SMCs. RhoA and Rho-kinase have also been identified in human penile tissues. Inhibition of Rho-kinase by Y-27632 in rats receiving NOS inhibitors (ie, l – N G -nitroarginine methyl ester hydrochloride [L-NAME]), showed an increase in intracavernosal pressure (ICP) that was independent of NO. Likewise, ICP was increased after adeno-associated viral gene transfer of a dominant-negative RhoA mutant in rat penile tissue. This indicates that the RhoA/Rho-kinase pathway regulates SMC contraction and maintenance of flaccidity. NO has been shown to inhibit the RhoA/Rho-kinase pathway in a crosstalk fashion. Likewise, the RhoA/Rho-kinase pathway may also help to regulate NO signaling.
Association of age-related changes in neuroregulatory factors with LUTS/BPH
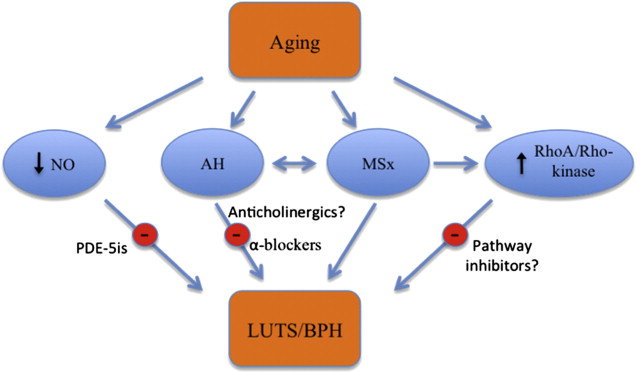
LUTS secondary to BPH is a common age-related complaint. Various population-based studies have confirmed this association, and it has been estimated that BPH affects 50% of men aged 50 years or older and 90% of men aged 80 years or older. BPH is a histologic definition that describes the nodular proliferation of both the stromal and epithelial elements within the transition zone of the prostate. LUTS secondary to BPH consists of both voiding and obstructive symptoms representing problems of bladder emptying and urine storage. Likewise, the prevalence of LUTS increases with age and it is found in 26% of men aged 40 to 50 years and 79% of men aged more than 70 years. Although the exact cause of the development of LUTS/BPH in aging men is not yet fully understood, alterations in neuronal function secondary to aging processes, such as MSx and autonomic hyperactivity, and age-related changes in neuroregulatory pathways, such as NO and RhoA/Rho-kinase, seem to be major contributors to this process ( Fig. 2 ).
MSx
MSx is a multifactorial disorder that has been associated with resistance to insulin-mediated glucose uptake. However, the exact mechanisms leading to the development of this syndrome are currently unknown. The Adult Treatment Panel III defines this condition as meeting 3 or more of the following criteria: abdominal obesity with waist circumference greater than 102 cm, hypertriglyceridemia greater than 150 mg/dL, high-density lipoprotein (HDL) cholesterol less than 40 mg/dL, blood pressure greater than 135/85 mm Hg, and fasting plasma glucose greater than 110 mg/dL. The prevalence of MSx has also been associated with age. MSx is found in 6.7% of those aged 20 to 29 years and increases to 42.0% of those aged 70 years or older. Multiple studies have shown that men with the components of MSx have a significantly increased risk of LUTS/BPH, larger prostate volumes, and faster annual BPH growth rate. In addition, it has been shown in animal models that long-term hyperglycemia causes neuronal cell death. This apoptosis seems to favor parasympathetic rather than sympathetic neurons. Subsequent increased sympathetic tone, also known as autonomic hyperactivity, may contribute to the development of LUTS in MSx (discussed later).
Autonomic Hyperactivity
MSx may help explain the connection between alterations in neuronal function, aging, and autonomic hyperactivity. Aging has been associated with an overall increase in autonomic tone, and there is a positive association between age and levels of circulating norepinephrine. Autonomic hyperactivity is a component of MSx and involves dysregulation of parasympathetic and sympathetic tone. In addition, increased autonomic activity in aging rats has been associated with the development of prostatic hyperplasia. Specifically, studies with spontaneously hypertensive rats showed that these rats were more likely to develop both increased autonomic activity and prostatic hyperplasia. These rats voided more frequently, had increased sympathetic innervation to the bladder, and had more low-volume spontaneous bladder contractions.
Epidemiologic studies have also elucidated the association between increased autonomic tone and BPH. Meigs and colleagues showed that symptomatic BPH is more likely to be associated with sympathetic activation as approximated by increased serum markers of heart disease, increased β-blocker use, and sedentary lifestyle. McVary and colleagues also examined markers of autonomic nervous system activity in men with LUTS secondary to BPH and International Prostate Symptom Scores (IPSS) of greater than or equal to 8. ANS activity was measured by heart rate, blood pressure, tilt table test response, and plasma and urinary catecholamines. There was a significant positive association between ANS hyperactivity and LUTS and prostate size. It seems that sympathetic nervous system tone contributes to the development of BPH in aging men.
NO
BPH has been associated with a decrease in the nitrinergic innervation of the prostate. The prostates of older rabbits were shown to have less NO-mediated relaxation and decreased overall nitrinergic innervation compared with younger counterparts. Bloch and colleagues examined prostatic tissue of men with symptomatic BPH and found a qualitatively decreased density of nitrogenic nerves compared with controls by measuring nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d), a marker for NOS expression. Similarly, it has been shown that there is decreased expression of the NOS gene in transurethral resection of prostate tissue samples of men with BPH compared with normal prostate tissue. The results of this study suggest that older men and those with larger prostates had significantly less NOS expression then younger men with smaller prostates.
RhoA/Rho-Kinase
Given its role in the regulation of muscle activity in both the bladder and prostate, it is likely that age-related alterations in this system could contribute to the development of urinary symptoms. The RhoA/Rho-kinase pathway may contribute to the development of LUTS/BPH, because inhibition of Rho-kinase prevents noradrenergic-induced contractions and proliferation of prostate SMCs. This bladder outlet obstruction caused by SMC proliferation may cause hypertrophy of the bladder and worsening of urinary symptoms. Support from this is derived from the increased RhoA and Rho-kinase expression and decreased MLCP activity in hypertrophied bladders. In addition, rat bladders that were denervated and hypertrophied, but not obstructed, showed that the effect of a muscarinic agonist was decreased by Rho-kinase inhibition. However, in normal bladders, Rho-kinase inhibition reduced the potency of carbachol but did not affect maximal contraction. Diabetes has also been shown to affect the RhoA/Rho-kinase pathway in the bladder. Animal models with diabetic rabbits show higher Rho-kinase expression, increased MLC phosphorylation, and decreased effectiveness of the Rho-kinase inhibitor Y-27632 on levels of MLC phosphorylation in the bladder. Taken together, these data suggest that the imbalance in the RhoA/Rho-kinase pathway may also influence LUTS.
Association of age-related changes in neuroregulatory factors with LUTS/BPH
LUTS secondary to BPH is a common age-related complaint. Various population-based studies have confirmed this association, and it has been estimated that BPH affects 50% of men aged 50 years or older and 90% of men aged 80 years or older. BPH is a histologic definition that describes the nodular proliferation of both the stromal and epithelial elements within the transition zone of the prostate. LUTS secondary to BPH consists of both voiding and obstructive symptoms representing problems of bladder emptying and urine storage. Likewise, the prevalence of LUTS increases with age and it is found in 26% of men aged 40 to 50 years and 79% of men aged more than 70 years. Although the exact cause of the development of LUTS/BPH in aging men is not yet fully understood, alterations in neuronal function secondary to aging processes, such as MSx and autonomic hyperactivity, and age-related changes in neuroregulatory pathways, such as NO and RhoA/Rho-kinase, seem to be major contributors to this process ( Fig. 2 ).
MSx
MSx is a multifactorial disorder that has been associated with resistance to insulin-mediated glucose uptake. However, the exact mechanisms leading to the development of this syndrome are currently unknown. The Adult Treatment Panel III defines this condition as meeting 3 or more of the following criteria: abdominal obesity with waist circumference greater than 102 cm, hypertriglyceridemia greater than 150 mg/dL, high-density lipoprotein (HDL) cholesterol less than 40 mg/dL, blood pressure greater than 135/85 mm Hg, and fasting plasma glucose greater than 110 mg/dL. The prevalence of MSx has also been associated with age. MSx is found in 6.7% of those aged 20 to 29 years and increases to 42.0% of those aged 70 years or older. Multiple studies have shown that men with the components of MSx have a significantly increased risk of LUTS/BPH, larger prostate volumes, and faster annual BPH growth rate. In addition, it has been shown in animal models that long-term hyperglycemia causes neuronal cell death. This apoptosis seems to favor parasympathetic rather than sympathetic neurons. Subsequent increased sympathetic tone, also known as autonomic hyperactivity, may contribute to the development of LUTS in MSx (discussed later).
Autonomic Hyperactivity
MSx may help explain the connection between alterations in neuronal function, aging, and autonomic hyperactivity. Aging has been associated with an overall increase in autonomic tone, and there is a positive association between age and levels of circulating norepinephrine. Autonomic hyperactivity is a component of MSx and involves dysregulation of parasympathetic and sympathetic tone. In addition, increased autonomic activity in aging rats has been associated with the development of prostatic hyperplasia. Specifically, studies with spontaneously hypertensive rats showed that these rats were more likely to develop both increased autonomic activity and prostatic hyperplasia. These rats voided more frequently, had increased sympathetic innervation to the bladder, and had more low-volume spontaneous bladder contractions.
Epidemiologic studies have also elucidated the association between increased autonomic tone and BPH. Meigs and colleagues showed that symptomatic BPH is more likely to be associated with sympathetic activation as approximated by increased serum markers of heart disease, increased β-blocker use, and sedentary lifestyle. McVary and colleagues also examined markers of autonomic nervous system activity in men with LUTS secondary to BPH and International Prostate Symptom Scores (IPSS) of greater than or equal to 8. ANS activity was measured by heart rate, blood pressure, tilt table test response, and plasma and urinary catecholamines. There was a significant positive association between ANS hyperactivity and LUTS and prostate size. It seems that sympathetic nervous system tone contributes to the development of BPH in aging men.
NO
BPH has been associated with a decrease in the nitrinergic innervation of the prostate. The prostates of older rabbits were shown to have less NO-mediated relaxation and decreased overall nitrinergic innervation compared with younger counterparts. Bloch and colleagues examined prostatic tissue of men with symptomatic BPH and found a qualitatively decreased density of nitrogenic nerves compared with controls by measuring nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d), a marker for NOS expression. Similarly, it has been shown that there is decreased expression of the NOS gene in transurethral resection of prostate tissue samples of men with BPH compared with normal prostate tissue. The results of this study suggest that older men and those with larger prostates had significantly less NOS expression then younger men with smaller prostates.
RhoA/Rho-Kinase
Given its role in the regulation of muscle activity in both the bladder and prostate, it is likely that age-related alterations in this system could contribute to the development of urinary symptoms. The RhoA/Rho-kinase pathway may contribute to the development of LUTS/BPH, because inhibition of Rho-kinase prevents noradrenergic-induced contractions and proliferation of prostate SMCs. This bladder outlet obstruction caused by SMC proliferation may cause hypertrophy of the bladder and worsening of urinary symptoms. Support from this is derived from the increased RhoA and Rho-kinase expression and decreased MLCP activity in hypertrophied bladders. In addition, rat bladders that were denervated and hypertrophied, but not obstructed, showed that the effect of a muscarinic agonist was decreased by Rho-kinase inhibition. However, in normal bladders, Rho-kinase inhibition reduced the potency of carbachol but did not affect maximal contraction. Diabetes has also been shown to affect the RhoA/Rho-kinase pathway in the bladder. Animal models with diabetic rabbits show higher Rho-kinase expression, increased MLC phosphorylation, and decreased effectiveness of the Rho-kinase inhibitor Y-27632 on levels of MLC phosphorylation in the bladder. Taken together, these data suggest that the imbalance in the RhoA/Rho-kinase pathway may also influence LUTS.
Treatment of LUTS/BPH
The importance of neuronal influences on the development of LUTS/BPH is underscored by the various therapeutic strategies that target them. These interventions include α-adrenergic antagonists, anticholinergics, and PDE-5 inhibitors (PDE-5i).
α-Adrenergic Antagonists
Modulation of autonomic activity by α-receptor antagonism has shown benefit in the treatment of LUTS/BPH. The inhibition of adrenergic receptors seems to decrease SMC tone in the bladder and prostate, decrease the number of SMCs, and improve LUTS related to BPH. It is unclear whether these drugs primarily produce their benefit through a direct effect on the prostate and bladder or through indirect effects via the central nervous system. Both first-generation agents (eg, phenoxybenzamine) and second-generation agents (eg, prazosin, terazosin, doxazosin, and alfuzosin) have been well studied with favorable improvements in IPSS scores and urinary flow rates. This suggests a prominent role of autonomic activity in LUTS/BPH.
Anticholinergics
Traditionally, anticholinergics were contraindicated in LUTS secondary to BPH because of concern about the risk of developing acute urinary retention (AUR). However, anticholinergics have been shown to be safe in men with LUTS. Although anticholinergics improve the symptoms of urgency and frequency, the effectiveness of these medications in the treatment of primarily obstructive symptoms has not been clearly shown. In addition, some studies have examined the role of cholinergic neuromodulation in the development of LUTS in aging men from studying the effects of botulinum toxin. Specifically, botulinum neurotoxin prevents the presynaptic release of ACh. By blocking the release of ACh, this intervention acts to chemically remove cholinergic input. Many studies have shown improvement in LUTS after intraprostatic botulinum injections. However, large, randomized, placebo-controlled studies are needed to confirm these results. Taken together, these studies suggest that cholinergic innervation may contribute to prostate growth and the development of LUTS, but future research is required.
PDE-5 Inhibitors and NO
Decreased nitrinergic innervation and NOS expression in the aging prostate suggest that the NO pathway may also contribute to the development of LUTS secondary to BPH. Studies have shown that targeting this pathway improves LUTS. For example, Klotz and colleagues showed in an open-labeled trial that isosorbide dinitrate in patients with heart disease and LUTS improves IPSS, decreased peripheral vascular resistance, and increase in peak flow rates.
Subsequent studies examined the effects of PDE-5 inhibitors (PDE-5i) on LUTS/BPH. These pharmacologic agents work by preventing the degradation of cGMP by PDE-5, leading to improved SMC relaxation. Several studies have also shown that sildenafil, a PDE-5i, improves LUTS. For example, McVary and colleagues examined the effects of sildenafil in men with ED and LUTS in a randomized, double-blind, placebo-controlled trial. Men treated with a 12-week course of the drug experienced a mean decrease in IPSS of 6.32 compared with a decrease of 1.93 in the placebo group. McVary and colleagues also examined the effects of tadalafil in a randomized, double-blind, placebo-controlled trial in 281 men with moderate to severe LUTS. At 6 weeks, the treatment group showed a significant mean change in IPSS score of −2.8 compared with −1.2 in the placebo group.
Similarly, Kaplan and colleagues showed the efficacy of combination sildenafil and alfuzosin in men suffering from LUTS and ED in an open-labeled study. The combination group had the greatest improvement in IPSS score from an initial mean of 17.8 (± 4.7) to 13.5 (± 4.2), representing a −24.1% change, compared with a change of 17.3 (± 4.3) to 14.6 (± 3.7) in the alfuzosin group and 16.9 (± 4.1) to 14.9 (± 4.2) in the sildenafil groups, representing decreases of 15.6% and 16.9% respectively. The ability of PDE-5is to improve LUTS suggests that the NO pathway contributes to the development of LUTS in aging men.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





