Fig. 17.1
A sample of Quadview image overview
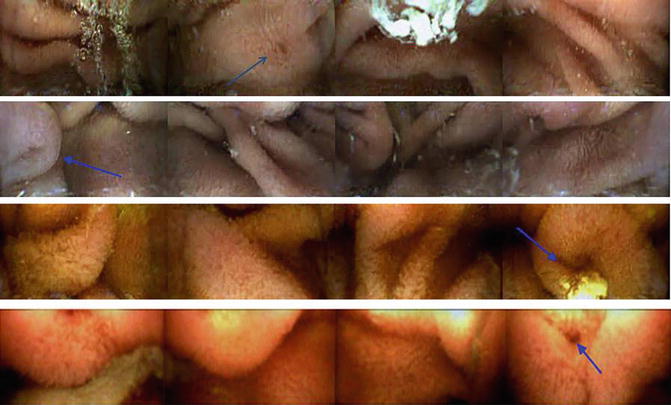
Fig. 17.2
Panoramic images from the CapsoCam capsule. Blue arrows, sample duodenal papillas. Reprinted with permission from Friedrich K, Gehrke S, Stremmel W, Sieg A. First clinical trial of a newly developed capsule endoscope with panoramic side view for small bowel: a pilot study. J Gastroenterol Hepatol. 2013;28(9):1496–501
There are limited comparative data assessing the diagnostic outcome of the various image-viewing options. Zheng et al. compared detection rates of 24 clips analyzed by 23 experienced endoscopists in four different modes. Single view at 15 and 25 frames per second (fps) in addition to Quad view at 20 and 30 fps. With the exception of the single view at 25 fps, overall detection rate was not affected by viewing mode or speed, and was independent of endoscopist experience. The authors recognize the limited pathology in their sample, the absence of surgical or endoscopic confirmation, and small sample size [9].
Given PillCam Progress Indicator
Upon identification of the duodenal and cecal landmarks, the Progress Indicator (Fig. 17.3) allows for a graphical indication of the percentage of small bowel that has been viewed at any point. It also estimates a degree of image similarity based on pixel redundancy between adjoining images, which aims to reflect the variable speed of transit of the capsule at various anatomic locations.
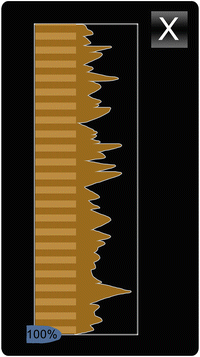

Fig. 17.3
Progress Indicator, PillCam® Capsule Endoscopy
Size Estimation
Various tools have been developed to approximate the size of lesions visualized on VCE. Validation of such systems is limited to specific lesions and cannot be generalized (e.g., Given’s Polyp Size Estimation tool). Most companies emphasize these tools as research platforms that should not be used in clinical decision-making.
Software Augmentation—Blood Indicators
Both Given and Olympus have developed software algorithms that highlight images containing red pixels. Indicative of suspected bleeding or angioectasias, they allow the interpreter to rapidly review sequences when capsules are utilized for obscure gastrointestinal bleeding.
At best complimentary, these features do not replace a thorough evaluation of all the images. There has been a conflicting wealth of literature regarding the accuracy of blood indicator software. The strongest evidence comes from active small bowel bleeding. Accuracy was higher in patients who required larger amounts of blood transfusion [10]. In a retrospective review of 109 lesions from a single center, the blood-indicating algorithm had a sensitivity, positive predictive value, and accuracy of 81.2 %, 81.3 %, and 83.3 % respectively [11], whereas other groups have deemed the technology to have no timesaving utility and limited clinical value [12]. Supporting that view, Signorelli et al. found an overall and per-patient sensitivity of 28 % and 41 % in their retrospective review of 95 patients [13]. Experimental ex-vivo models indicate that yield is greatly affected by background color and capsule velocity [14].
To avoid mislabeling images, such features can only be activated once the anatomic landmarks have been identified. Moreover, the threshold for degree of redness and number of pixels can be adjusted. In the Given system the suspected bleeding is displayed as red “ticks” overlying the time bar (Fig. 17.4). A review method is available allowing easy navigation between the suspected images.


Fig. 17.4
Blood Indicator, PillCam® Capsule Endoscopy
Blood detection is currently only available for small bowel capsules, but the technology should be easily exportable to colonic and esophageal systems with limited tweaking.
Resolution and Image Adjustment
Moving away from electronic parts designed for general consumer use, biomedical engineering collaborators have developed capsule technology further. This includes, but is not limited to, photo recording chips with greater dynamic range that can switch between linear and logarithmic to better emulate the natural accommodation and range of the human eye. Such capsules are advancing the frontier with images of greater resolutions, captured at faster frame rates, with minimal power consumption [15].
Some manufacturers include proprietary technology to enhance images by altering sharpness, color, brightness, and contrast [16]. One such innovation, virtual chromoendoscopy, employed by Fujinon’s Intelligent Chromoendoscopy and Given’s FICE (versions 1, 2 and 3) and Blue Mode, use spectral estimation to narrow the bandwidth of conventional endoscopy. These filters are analogous to digitized formats of the narrow band imaging of conventional endoscopy. There are widely discrepant results in the literature regarding the utility of these modalities. In a review assessing the validity of FICE or Blue mode, improvement ranged from 7.7 % to 87.7 % depending on the indication and mode of FICE. Moreover, in the same review FICE false positivity was increased with poor bowel preparation [17].
Olympus’s Contrast Image Capsule has combined hardware and software changes which utilizes a blue enhanced white light emitting diode. In addition to white light images, contrast images are generated by extracting the green and blue wavelengths from the spectrum [18]. A feasibility study limited to a few patients suggests that this technology enhances visibility in polyposis syndromes [19].
In an editorial, Spada and associates reviewed the discordant literature and concluded that virtual chromoendoscopy visually enhanced lesions which had been previously identified, but did not improve upon the detection rate [20].
Panoramic and 3D Modeling
Panoramic segmental images of the small bowel can be captured by positioning cameras on the side of the capsule (CapsoCam, Capsovision, CA). Similar images can also be generated via mathematical modelling. Moreover, algorithmic manipulation of a series of two-dimensional images has generated a three-dimensional model of the small bowel as reported by Fan et al. [21]. Such software reconstruction significantly enhances vascular lesions but has been shown to have limited yield in inflammatory and protruding lesions [22].
Localization
Triangulation by utilizing the variable strength of signals reaching the various leads allows for the approximation of the location of the capsule in the small bowel, which can be modeled on a two-dimensional chart. The belt and sensor array can be used for the location of the Given PillCam small bowel and colon (Fig. 17.5) and the Olympus Endocapsule systems. Localization is of greatest yield in anatomical anchored segments of the bowel.
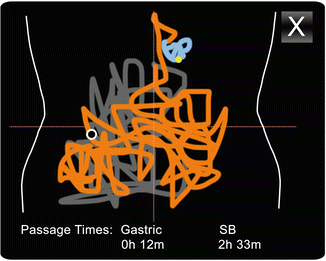

Fig. 17.5
Localization, PillCam® Capsule Endoscopy
Some software systems allow for the segmentation of the figure to include various anatomical locations, usually reported by a number of colors. Under development, feature tracking [23], contraction pattern analysis [24], and locality-preserving projections [25] have generated a precision of 95 %, 51 %, >90 % respectively in automatic localization of images relative to gastrointestinal organs.
Other modalities include the use of embedded magnetoresistive arrays and ultrasonic localization.
Reporting
Most software packages have sophisticated reporting modules. Results are documented alongside indications and performance parameters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








