Fig. 2.1
Esophago-digiunal anastomosis after total gastrectomy: normal endoscopic findings
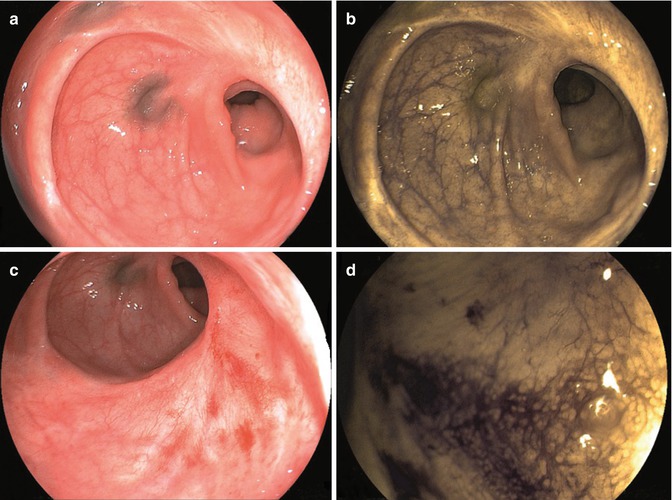
Fig. 2.2
Lower colonic anastomosis with a small reduction in caliber (a). Electronic chromoendoscopy (FICE-system evaluation) with negative findings (b). Slight hyperemia is visible at the edge of the anastomosis (c). Electronic chromoendoscopy confirmed negative findings (d)
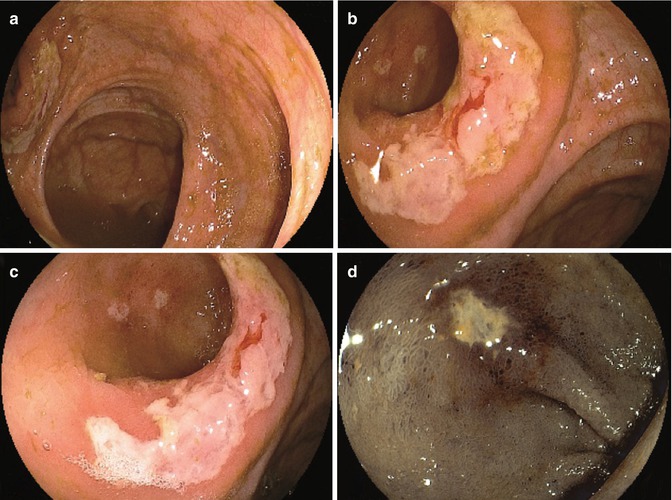
Fig. 2.3
Ileocolonic (side-to-side) anastomosis after right hemicolectomy (a). Whitish discoloration involving half of the anastomotic border (b). Conventional close-up view with evidence of superficial erosions of the ileal mucosa (c). Electronic chromoendoscopy (FICE system) of the ileal erosions with negative histologic findings (d)
A significant reduction of the intestinal lumen, even if asymptomatic, should be described and monitored, while in case of intestinal stenosis, fistula, or dehiscence, other imaging modalities together with prompt treatment should be scheduled and selected among the different options (endoscopic dilation, stent placement, or surgical reconstruction) (Fig. 2.4).
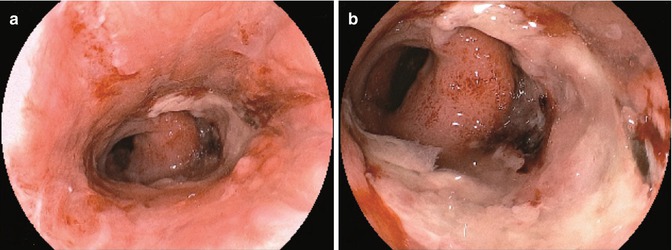

Fig. 2.4
Coloanal anastomosis with dehiscence and large amount of fibrin deposit (a). Close-up view with evidence of anastomotic leakage and necrotic area (b)
2.3 Oncological Criteria of Follow-Up
Endoscopists should keep in mind clinical criteria for an accurate follow-up of the patient: synchronous cancer is defined as a cancer detected within 1 year of follow-up, while metachronous cancer is that one detected after 1 year of follow-up, while concomitant cancers are defined as multiple cancers detected before the surgical treatment. In this setting, we define the miss rate as the proportion of missed cancer out of all synchronous cancer [7]. These parameters have been introduced in order to better define the oncological criteria of the endoscopic follow-up. Timing of the first endoscopic evaluation has been questioned as a risky procedure particularly when dealing with difficult anastomosis such as esophageal ones. Maish [8] reports as early endoscopy after esophagectomy provided reliable identification of graft ischemia in 63 over 102 patients of his series. Upper flexible endoscopy performed a median of 9 days after operation was safe and with no anastomotic injuries. In another UK series [9] esophagoscopy was attempted within 1 week of esophagectomy in order to check the anastomosis and the reconstructed stomach of 79 consecutive patients. A total of 15 patients with gastric ischemia, two with a leak, and four with ischemia and leakage were detected, thus confirming endoscopy as a safe and accurate procedure. Intraoperative endoscopic diagnosis has been questioned to evaluate circular-stapled colorectal anastomosis during laparoscopic surgery and as a possible resource to prevent bleeding and possible leakage [10]. The patients with and without routine intraoperative endoscopic assessment were compared regarding postoperative complications, and even if the postoperative rate of bleeding and leakage was not significantly reduced, intraoperative endoscopy was accurate in the early detection and treatment of these complications. The implementation of new imaging modalities such as dye-spraying technique, virtual chromoendoscopy, and high-resolution endoscopy not only in eastern countries increases the early detection of neoplastic disease. These techniques made a much accurate diagnosis of neoplastic disease possible even in the endoscopic follow-up of surgically treated patients, so far improving the early detection of neoplastic recurrence. Endoscopic surveillance with chromoendoscopy in a Japanese series of 97 colectomized with ileorectal anastomosis ulcerative colitis showed definite dysplasia in four patients, who received IRA; among them two were adenocarcinoma with submucosal invasion [11, 12]. Postoperative surveillance endoscopy performed by an experienced endoscopist and with dye-spraying technique was useful to detect cancer at an early stage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








