, Mark Thomas1 and David Milford2
(1)
Department of Renal Medicine, Birmingham Heartlands Hospital, Birmingham, UK
(2)
Birmingham Children’s Hospital, Birmingham, UK
Abstract
In this chapter we explain:
When immunology tests are most helpful
How diagnostic information is gained from measuring:
Immunoglobulins and free light chains
Anti-nuclear antibody
Complement components C3 and C4
Anti-neutrophil cytoplasmic antibody (ANCA)
Anti-glomerular basement membrane antibody
Anti-phospholipase A2 receptor antibody
Do the Right Test on the Right Patient
The kidney is vulnerable to damage by the immune system [1]. The high blood flow rate through glomeruli exposes them to immunologically active cells. Antibodies and lymphocytes can attack vascular endothelial cells. Antigen-antibody complexes and complement molecules are trapped within the filtration barrier and taken up into mesangial cells and podocytes by endocytosis. Antibodies can be generated against antigens expressed in the glomerular basement membrane or on podocytes. Cell injury, inflammation and repair responses cause a wide range of kidney diseases.
Immunological tests on serum can help distinguish between conditions with clinically similar presentations. It is tempting to tick a number of boxes on the blood test request form to check for immune-mediated disease – a kidney disease ‘immunology screen’. However, this may not be a wise move.
There is a danger of a false positive result with all screening tests. The likelihood that a positive result will be ‘false’ is dependent upon the pre-test probability – the prior odds – that the patient actually has the disease in question.
For example, the chances of an anti-nuclear antibody (ANA) test being positive in a healthy older person can be as high as one in five. The chances that someone with slowly progressive chronic kidney disease with minimal proteinuria has systemic lupus erythematosus (SLE) are perhaps one in a thousand. So a positive ANA test in such a person has a 1000/5 = 200 to 1 chance of being a false positive.
Similarly, serum IgA levels are elevated in a high proportion of patients with IgA nephropathy . A raised level can be useful supportive evidence in a patient with other typical features of IgA nephropathy. However, serum IgA is elevated in many other conditions including chronic infection and so on its own is not diagnostically specific [2].
The risk of ignoring these laws of probability is that you will be led along a path of ever-more invasive tests to exclude a serious cause for the abnormal result. For example, immunoglobulins and a protein electrophoresis are often assayed in patients with chronic kidney disease to ‘exclude’ multiple myeloma. The finding of a monoclonal paraprotein raises the possibility that the paraprotein is the cause of the kidney disease and the patient would benefit from chemotherapy. In reality, it is more likely that the paraprotein is unrelated to the kidney disease, a monoclonal protein of undetermined significance (MGUS). You may be left with the dilemma of whether to exclude monoclonal protein-related kidney disease by performing a renal biopsy.
The following sections describe the most commonly used immunological tests with the clinical features that indicate when they are likely to have diagnostic value.
Immunoglobulins , Protein Electrophoresis , Free Light Chains in Serum and Urine
Clinical features:
1.
Multiple myeloma with myeloma kidney
Anaemia
Deteriorating GFR
Minimal proteinuria on dipstick testing but raised urine protein:creatinine ratio
Bone pain with normal alkaline phosphatase
Hypercalcaemia
2.
Amyloidosis
Nephrotic-range proteinuria or nephrotic syndrome
Hypotension
Heart failure
Bruising
Free lights chains are smaller than albumin and freely filtered by glomeruli. Their serum concentration is therefore dependent upon GFR and patients with reduced GFR have increased serum levels of polyclonal free light chains. The levels are further increased by inflammation and are associated with an increased risk of progression of chronic kidney disease and of mortality [3].
The ratio of kappa to lambda light chain concentrations is used to identify a monoclonal excess of one type of chain. An abnormal ratio is not proof of clonality; further tests are needed to prove a monoclonal plasma cell disorder.
Monoclonal free light chains in the urine (Bence Jones protein) are not detected by dipstick testing but the laboratory urine protein assay does detect them. A specific test for free light chain is the most sensitive.
Multiple Myeloma
The kidneys can be damaged in patients with myeloma through a range of mechanisms. Hypercalcaemia has a direct effect on GFR (see Sect. “Hypercalcaemia” in page 189). Filtered free light chains are reabsorbed by proximal tubular cells and can cause cell injury. If light chains are in large amounts, they may combine with uromodulin to form casts that obstruct the tubules – ‘cast nephropathy’ .
It is helpful to compare changes over time in the amount of monoclonal protein in serum with the eGFR. If the two mirror each other, the monoclonal protein is more likely to be causing the decline in GFR and so be a monoclonal protein of renal significance (MGRS) rather than of undetermined significance (MGUS) [4] (Patient 14.1).
Patient 14.1: Myeloma Kidney
Mrs. Drake suffered repeated relapses of her myeloma over the course of four years, shown by rises in the concentration of monoclonal protein (Fig. 14.1).
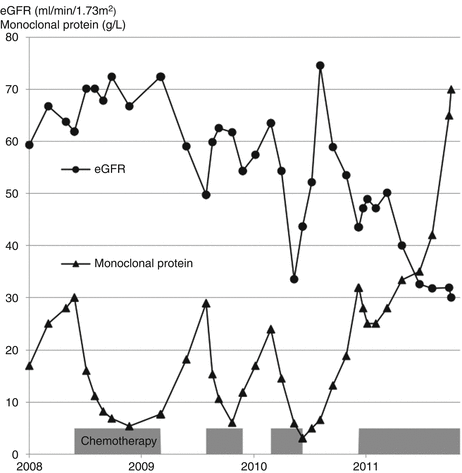

Fig. 14.1
eGFR mirrors the rises and falls in the concentration of monoclonal protein in a patient with multiple myeloma
During the first relapse in 2008 her kidney function was not affected.
During the second and third relapses in 2009 and 2010 her eGFR declined as the paraprotein level rose. Both times, further treatment led to a decline in paraprotein and recovery in eGFR.
Sadly her disease continued to progress and became unresponsive to treatment after 2010. She died in 2011.
Amyloidosis
Systemic amyloidosis refers to a group of conditions in which a small subunit of a plasma protein undergoes polymerisation and is deposited in the tissues as a fibril with a beta-pleated sheet configuration.
Examples of the proteins that can form amyloid deposits are:
Immunoglobulin light chain (25 kDa): AL amyloidosis in plasma cell dyscrasia
Serum amyloid A (12 kDa): AA amyloidosis in chronic inflammation
Beta-2 microglobulin (12 kDa): Dialysis amyloidosis
The kidneys can be affected by deposition of amyloid protein into vessel walls and glomerular membranes (Figs. 14.2 and 14.3). This disrupts the filtration barrier allowing proteinuria that is often severe enough to cause the nephrotic syndrome .
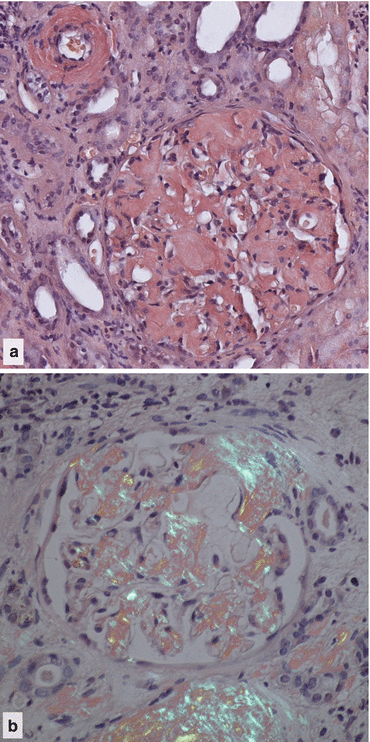
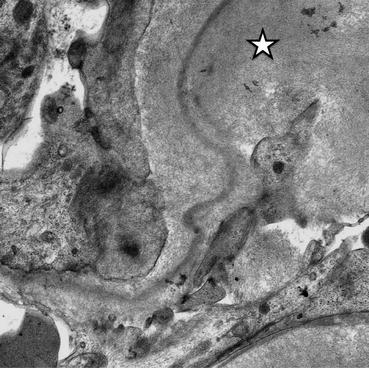

Fig. 14.2
Light micrographs of a section of a renal biopsy from a patient with amyloidosis stained with Congo red . (a) Amyloid, coloured red, is deposited throughout the glomerulus and in the wall of an arteriole (top left). (b) anomalous colours are displayed when the specimen is examined between a crossed polarizer and analyser [5]. ×200

Fig. 14.3
Electron micrograph showing amyloid fibrils (star) deposited in the glomerular basement membrane
Amyloid protein may also affect systemic arteriolar walls and the autonomic nerve fibres that mediate vasoconstriction. This causes postural hypotension and can reduce kidney blood flow.
Anti-nuclear Antibody , Anti-dsDNA Antibody
Clinical features:
Systemic lupus erythematosus (SLE) nephritis
Nephritic syndrome :
Proteinuria ++ or greater
Microscopic ++ or greater
Macroscopic haematuria (see Fig. 11.2)
Worsening GFR over weeks or months
About half of people with SLE have kidney involvement, this proportion being higher in men and in black races. The pattern of kidney disease is very variable, ranging from asymptomatic urine abnormalities to acute nephritis and nephrotic syndrome . A biopsy is required to characterise the type of kidney disease.
Histological appearances are grouped into six classes, each associated with different clinical features and requiring different treatment [6].
Complement C3 and C4
Clinical features:
Glomerulonephritis
Nephritic syndrome :
Proteinuria ++ or greater
Microscopic ++ or greater
Macroscopic haematuria (see Fig. 11.2)
Worsening GFR over days, weeks or months
Components of the complement cascade (Fig. 14.4) are consumed in a range of auto-immune disorders.
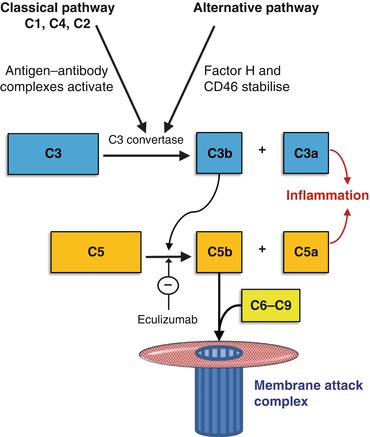

Fig. 14.4
The main elements of the complement cascade. Immune complexes activate the classical pathway, lowering levels of both C3 and C4. When the alternative pathway is activated, typically C3 is low but C4 is normal. Factor H and CD46 inhibit the alternative pathway C3 convertase. If they are defective, the alternative pathway is overactive and leads to cell damage by the generation of membrane attack complexes (C5b-9). Eculizumab blocks C5 cleavage and is used to control overactivity of the alternative pathway in atypical haemolytic uraemic syndrome (aHUS) (see Sect. “Kidney disease linked to causes of anaemia” in page 176)
Immune complexes of antibody and antigen are deposited in the glomeruli where they can fix complement and cause glomerulonephritis. Deposits of the C3 component of complement are the common feature in a recently characterised category of glomerulonephritis [7].
Serum C3 and C4 levels are reduced in patients with nephritis associated with SLE and cryoglobulinaemia . This suggests activation of the classical and alternative pathways. C4 levels may be unreliable for monitoring SLE nephritis because genetic deficiencies of C4 are relatively common and cause a low C4 without disease.
C3 is usually decreased more than C4 in patients with C3 glomerulopathy , Dense Deposit Disease , post-streptococcal glomerulonephritis , bacterial endocarditis glomerulonephritis and cholesterol crystal embolism . This suggests activation of the alternative pathway.
The drop in C3 found in children with post-streptococcal glomerulonephritis is usually short lasting. If the level of C3 remains low for longer than eight weeks, the autoantibody ‘C3 nephritic factor’ that blocks the regulation of C3 activation may be present [8].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








