Fig. 7.1
Bilateral weak radiopaque staghorn stones in a patient with cystinuria. The patient was referred with bilateral JJ stents
7.2.1.2 Intravenous Urography (IVU)
Traditionally, IVU was the preferred imaging modality for both diagnosis of the stone disease and planning of treatment including access in PNL. With use of both anterior-posterior (AP) and oblique views, IVU presents the anatomy of the collecting system as well as its relationship to the ribs, thereby predicting the need for a supracostal access [1]. IVU is usually performed in the supine position. If a prone position or a modified supine position is chosen for PNL, this information may be of limited value, since changing the position also modifies the anatomic interrelations. For instance, it has been shown that by changing from the prone to the prone-flexed position, a supra-11th rib access may be converted to a supra-12th rib and a supra-12th to an infracostal access with obvious advantages when it comes to potential complications [3, 4]. Furthermore, in supine positions a supracostal access is rarely needed.
7.2.1.3 Computerized Tomography (CT)
Non-contrast CT (NCCT or CT KUB) unequivocally performs significantly better than IVU (level of evidence 1a) in the evaluation of acute flank pain and the diagnosis of urolithiasis [5, 6], and indeed in many institutions IVU is no longer available [1]. Both sensitivity and specificity of NCCT in the evaluation of renal and ureteral calculi approach 100 % [7].
However, with regard to anatomical information, NCCT is a poor substitute for IVU, and most patients undergoing PNL require additional anatomical imaging after an otherwise diagnostic NCCT [8]. This may be achieved by a retrograde pyelography at the time of surgery, which in many instances may be enough to delineate the collecting system sufficiently for a safe puncture. Standard CT urography, which has been proposed as the ‘catch-all’ diagnostic procedure for all renal tract anomalies [9], has its own limitations for calculus management, since excretory phase studies (ECT) may mask calculi [8]. Whether three-dimensional (3-D) CT pyelography offers any value over conventional IVU and NCCT has been questioned [1]. We will argue, however, that multidetector CT pyelography with multiplanar reconstruction and 3-D reformatting (3-D CT) is highly valuable in cases with complex anatomy/stone burden in which you anticipate surgical difficulty. A 3-D CT not only presents exact volume, orientation and location of stone(s) in relation to the collecting system, thereby facilitating selection of the optimal calyx for percutaneous access (Fig. 7.2), it also provides excellent perirenal organ mapping in combination with the CT images used for the 3-D reconstruction, thereby presenting the optimal plane of access in order to avoid injury of adjacent organs such as the liver, spleen, colon and pleura, which is of special value in patients with unusual body habitus (Fig. 7.3) and renal anomalies (Fig. 7.4). A 3-D CT demonstrates accurately the presence of parallel calculus-bearing calyces, and it displays calyceal orientation, thickness of narrowed calyces or the neck of a calyceal diverticulum [8, 10]. In special situations this may be highly valuable, when deciding the best route of access and when performing Endoscopic Combined IntraRenal Surgery (ECIRS) in order to be guided around ‘from above and below’ in very complex systems [11]. Previously, 3-D CT was considered time-consuming and its reconstructions unreliable and therefore unsuitable for routine use [12]. Recent studies using interobserver statistics have, however, confirmed that 3-D CT in routine daily clinical practice is applicable, visualizing clinically significant calculi and accurately reconstructing the upper tract in rich 3-D format [8]. We are currently using this imaging modality in patients with renal anomalies and anticipated access difficulties, such as horseshoe kidneys (Fig. 7.5), malrotated kidneys, pelvic kidneys and calyceal diverticulum stones (Fig. 7.6). In the Clinical Research Office of the Endourological Society (CROES) PCNL Global Study, it was shown that PNL in anomalous kidneys was just as successful with regard to stone-free rates as PNL in normal kidneys [13]. Median operative time was significantly longer, however, and access for PNL was unsuccessful in significantly more patients in whom renal anomalies were present. In our experience access difficulties in such cases may be limited and consequently operative time reduced using preoperative 3-D CT. CT has been considered inferior to IVU when evaluating stone-bearing calyceal diverticula [1]. As demonstrated in Figs. 7.6 and 7.7 it is clear, however, that 3-D CT is capable of delineating the collecting system anatomy including diverticulum size, location and stone burden excellently, and we believe that 3-D CT ultimately will replace IVU for preoperative access planning in patients with suspected renal anomalies, also in cases presenting with calyceal diverticula.
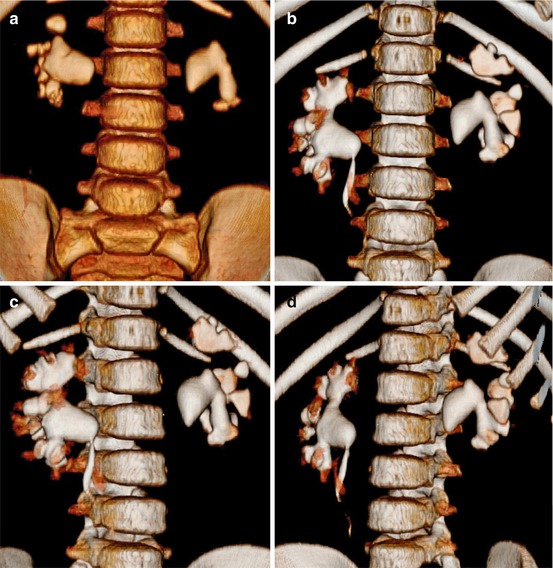
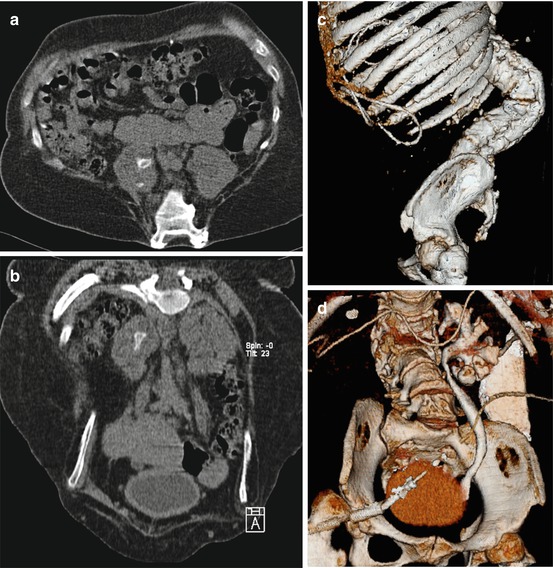
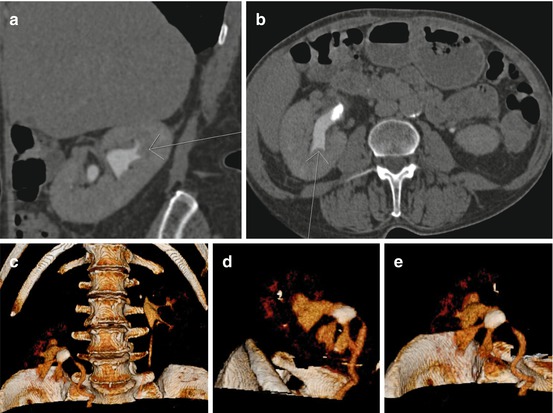
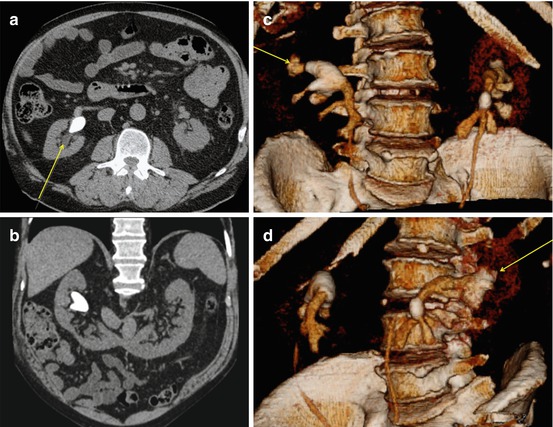
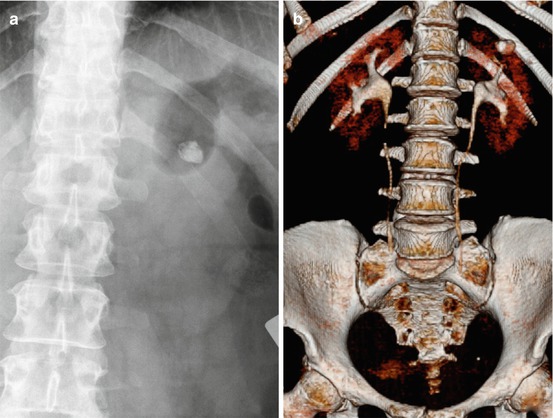
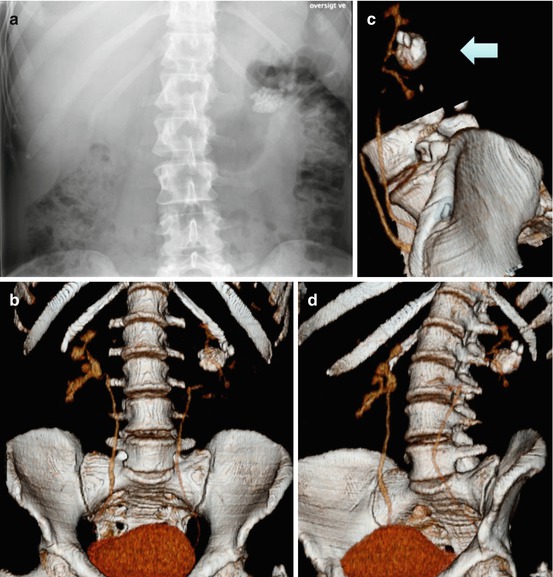

Fig. 7.2
Three-dimensional (3-D) reconstruction of bilateral staghorn stones. (a) Non-contrast CT (NCCT); (b–d) excretory CT (ECT). The 3-D reconstruction details the stone volume in relation to the collecting system from different angles, thereby guiding the optimal access

Fig. 7.3
Patient with severely altered body habitus due to myelomeningocele. (a) Axial reconstruction showing spina bifida and medially located kidneys with a pelvic and a calyceal stone in the right kidney; (b) coronal reconstruction showing the right-sided stone in the renal pelvis, note the malformed columna pointing towards you at top of the image; (c) 3-D lateral view showing the relation of the kyphoscoliosis to the ribs; (d) 3-D reconstructed excretory phase, note the ventriculoperitoneal shunt catheter

Fig. 7.4
Coronal (a) and axial (b) views of a kidney located just at the entry of the bony pelvis. Arrows show safe puncture line away from the liver and intestines. A 3-D reconstruction (c, d) from different angles demonstrating that an upper pole puncture will be most appropriate due to narrowing of the calyceal necks of the lower and middle calyces. Furthermore, the working space away from the bony pelvis makes the upper pole puncture most appealing (e)

Fig. 7.5
(a) Axial non-contrast CT (NCCT) of horseshoe kidney; (b) coronal NCCT of horseshoe kidney; (c, d) 3-D reconstruction at different angles. Arrows pointing at preferred access, which in horseshoe kidneys most often is the upper posterior calyx pointing directly backwards

Fig. 7.6
Left calyceal diverticulum stone on (a) KUB and (b) 3-D CT. The latter showing the exact relation of the diverticulum to the collecting system and the ribs

Fig. 7.7
Stones in a very large calyceal diverticulum demonstrated on (a) KUB, (b–d) in 3-D reconstructions at different angles delineating the narrow collecting system and its relation to the diverticulum at the middle calyx, as well as diverticulum size and stone burden. Access was performed by a direct puncture to the diverticulum straight from behind as indicated by the arrow (c)
In some centres a KUB is added to the initial CT examination to evaluate whether the calculi are radiopaque on fluoroscopy, which is important information, when access is performed and presence of residual fragments are evaluated during and after surgery. This is unnecessary exposure of the patient to ionized radiation; however, since it has been shown that if the calculi are visible on the CT planning image (CTI, scout, topogram, etc.), they are also visible on KUB/fluoroscopy (positive predictive value 100 %) [14].
7.2.1.4 Magnetic Resonance Imaging (MRI)
Although MRI has the ability to detect the secondary effects of obstructive urolithiasis [15], MRI is unreliable in identifying both renal and ureteral calculi, and at present MRI has no role in the preoperative evaluation of patients undergoing PNL. In case of younger patients, pregnancy and patients that have undergone multiple prior CT exams in which you want to avoid ionized radiation, ultrasound seems to be the better alternative.
7.2.2 Relationship of the Kidney to Adjacent Organs
In a comparative study of CTs performed in supine and prone position, particular attention was given to bowel found posteriorly to the kidneys (retrorenal colon): its frequency of occurrence on 500 scans of supine patients was 1.9 %, but 10.0 % in the 90 prone patients [16], suggesting that supine PNL bears a lower risk of colon injury. This rather high frequency of retrorenal colon in prone position was confirmed in another study, in which the prevalence of colon lying posterior to the kidney was 13.6 % on the right and 11.9 % on the left side in males, whilst in females it was 13.4 % on the right and as high as 26.2 % on the left side [17]. Based on these data it may be argued that the supine position appears to be favourable with regard to risk of colon injury. This is supported by the fact that no colon injuries have been reported in the literature with supine PNL and that visceral organ-to-percutaneous tract distance has been found to be shorter when patients are placed in the prone position on bolsters compared with the supine position [18]. These observations were made using axial images of NCCT studies, however, and it has been shown that the risk of colon injury is overestimated by evaluation of axial CT images compared with multiplanar reformatted images (3-D CT) [19]. It is unquestionable, however, that the supine position for PNL has the advantage that the relative orientation of the anterior and posterior calyces as well as the interrelations of the perirenal organs to the kidney on the CT image may be transferred directly to surgery, since preoperative CT is most often performed in the same position. Whether CT before prone PNL should be done in the prone position is still a matter of debate [1].
Upper pole punctures are rarely needed in supine PNL, since most complex calculi can be dealt using ECIRS with lower pole punctures [11]. If an upper pole supracostal puncture is planned, the relation of the percutaneous tract to the pleura and the lung has to be considered. A 3-D CT in inspiratory and expiratory phases may be helpful in demonstrating the anatomical relationships among the kidney, calculi, pleura, diaphragm and ribs [20]. Although the reported incidence of pleural transgression during supracostal access is variable, most investigators recommend percutaneous puncture while the patient is in expiration [1].
7.2.3 Estimate of Renal Function
In case of severely reduced overall renal function, contrast-enhanced imaging is contraindicated, due to risk of further worsening of renal function. Both IVU and contrast-enhanced CT, together with knowledge of plasma creatinine, give a rough estimate of renal function. If these examinations give suspicion of severely reduced function of the stone-bearing kidney, a renogram/scintigraphy is mandatory for exact evaluation of the renal split function. The split function threshold deciding whether the patient should undergo PNL or nephrectomy is depending on the total renal function, which must be measured by a clearance estimate (Fig. 7.8).
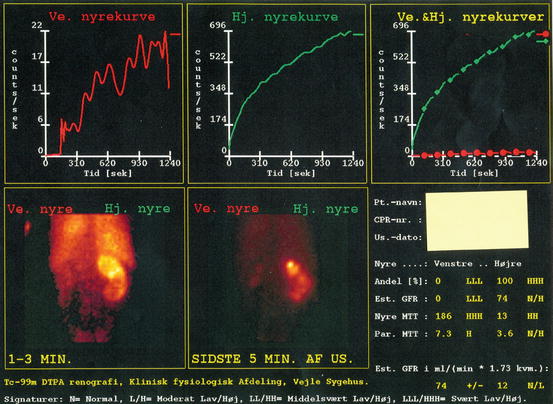

Fig. 7.8
Renogram in a patient with bilateral infection staghorn stones showing a nonfunctioning left kidney (red line) and compromised drainage of the right kidney (green line). The patient was treated by right-sided percutaneous nephrolithotomy (PNL) and left-sided laparoscopic nephrectomy
7.2.4 Estimate of Stone Fragility and Patient Selection
Traditionally, smaller kidney stones (<2 cm) may be approached by SWL. Numerous factors affect SWL outcome including obesity, anatomical anomalies of the patient in general and the urinary tract in particular, size and number of stones and stone location and composition. Several authors have studied the role of measuring Hounsfield units (HU) in order to predict crystalline composition of the stone as well as stone fragility in ESWL. Zarse et al. have addressed this elegantly by using high-resolution detection of internal structure of renal calculi by helical CT [21]. They found that by using a narrow slice width and bone windows greatly improved the visualization of kidney stone structure on helical CT. These results opened up for new possibilities for determining the relationship of stone structure and fragility for ESWL. They interestingly found that CT visible internal structure rather than HU of COM stones predicted lithotripsy fragility in vitro. COM stones of homogeneous structure required almost twice as many shock waves (SWs) to comminute than stones of similar mineral composition that exhibit internal structural features that were visible by CT. HU values of COM stones did not correlate with stone fragility. Thus, it seems that it is stone morphology, rather than X-ray attenuation, which correlates with fragility to SWs in this common stone type. This simple technique has proven to be an important clinical tool for the primary selection of patients to either ESWL or endoscopic treatment. This was further indicated in a study of cystine stones in which it was found that CT visibility of void regions in cystine stones was an indicator of fragility during ESWL. Conversely, cystine stones that appeared to be homogeneous by CT were likely to be resistant to SWs [22]. Thus, these findings may be used in the clinical setting for selecting patients for primary ESWL or primary endourological treatment such as PNL and thereby increasing efficacy of both treatment approaches (Fig. 7.9).
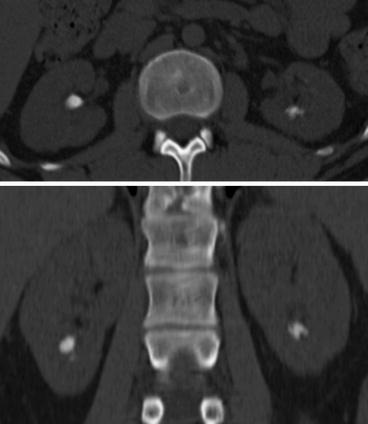

Fig. 7.9
Inhomogeneous stones in the lower calyces of a cystinuric patient. Traditionally, cystine stones are ESWL resistant; however, these inhomogeneous stones containing void regions were successfully treated by ESWL in two single sessions
7.3 Intraoperative Imaging
Intraoperative imaging is needed both for access and guidance of the endoscopic inspection of the collecting system [1]. The percutaneous puncture may be guided by biplanar fluoroscopy, ultrasound (US) and/or CT. Open configuration MRI may be used for nephrostomy placement in extraordinary cases such as very complex calculi in pregnancy in which ultrasound has failed or is considered unsuitable [23, 24].
It is important that whenever possible the access is carried out in the operating room (OR) at time of surgery, in order to eliminate transfer of the patient between different departments. The urologist alone can do the access. If the access is performed by an interventional uroradiologist, it should preferably be done in collaboration with the endourologist in a one-stage setting in the OR for selection of the optimal tract based on intrarenal anatomy and the ability to make secondary tracts [25]. Also placement of a ureteral catheter for contrast injection provides the ability to create a dilated system and presents intrarenal anatomic details, which improves the chances of getting ‘the perfect puncture’ through the cup of the desired calyx. Fewer access-related complications and higher stone-free rates can be achieved in this manner.
7.3.1 Fluoroscopy
Traditionally, biplanar fluoroscopy with a rotating C-arm has been the most common imaging modality for obtaining percutaneous access in PNL, and regardless the imaging modality used for access guidance, intraoperative fluoroscopy complementary to endoscopy is considered indispensable for successful and safe stone removal [1, 25]. Usually, a retrograde pyelography using a ureteral catheter with the C-arm in the vertical position in prone PNL and in the supine position with the C-arm tilted in accordance with degree of patient rotation is performed to delineate collecting system and locate stone(s) [25, 26]. Tilting the C-arm towards the surgeon (20°–30°) and in the caudal or cranial direction depending on whether the lower or upper pole is being accessed presents the desired posterior calyx – usually the posterior one – for end-on puncture [25]. In the prone position air instead of contrast may be instilled into the collecting system to present the posterior calyx without obscuring the stone [1]. When using contrast for puncture guidance, the posterior calyx will be less contrast enhanced in the prone position and more contrast enhanced in the supine position compared to the anterior calyces due to gravity [27].
During rigid and flexible nephroscopy, fluoroscopy injection of diluted contrast through the scope, when needed, enhances complete inspection/visualization of the collecting system including the ureter, thereby increasing stone-free rate [1].
7.3.2 Ultrasonography (US)
The limitations of using US alone for percutaneous puncture include difficulties in appropriate targeting of a non-distended calyx, poor image quality in obese patients, limited ability to identify anatomic details such a narrow infundibulum and difficulties in differentiating nephrocalcinosis from nephrolithiasis [1]. On the other hand, in combination with fluoroscopy US offers clear advantages. US permits direct real-time inspection of the intended percutaneous tract avoiding injury to any major intrarenal vessels or perirenal organs, while at the same time securing exact end-on puncture of the calyx [28]. The fact that fewer colon injuries have been reported with supine PNL may also in part be attributable to the combined US/fluoroscopy-guided puncture, which is a golden standard in this procedure [11, 26]. This combined access guidance approach also has been shown to eliminate organ perforation risk in prone PNL [28]. Indisputably, US is the preferred initial access guidance for pregnant and renal transplant patients and in patients in which a retrograde passage of a ureteral catheter for opacification of the collecting system is precluded [1].
7.3.3 Computerized Tomography (CT)
CT-guided access is reserved to very complex cases in which access has failed using standard fluoroscopy/US or in which special access difficulties are anticipated. Such indications may include spinal dysraphism, morbid obesity, abnormal visceral anatomy (retrorenal colon or spleen), urinary diversions, ectopic kidney, horseshoe kidney and transplant kidney [29]. Percutaneous renal access may be guided by CT using real-time CT or in combination with CT pyelography [28] (Fig. 7.10). Interventional CT suites are available but not yet in widespread use for PNL [24]. CT-guided puncture feasibly can be performed in a conventional CT room without radiation exposure to the interventionist. A laser guide and adjunctive guidance devices may ease the procedure [29, 30]. The major advantage of CT-guided puncture is the ability for excellent real-time imaging of perirenal organs, avoiding organ injury in patients with very complex anatomy. Also, performing PNL in interventional CT suites will enable you to evaluate presence of residual fragments with the best available imaging modality, which potentially will increase stone-free rate.
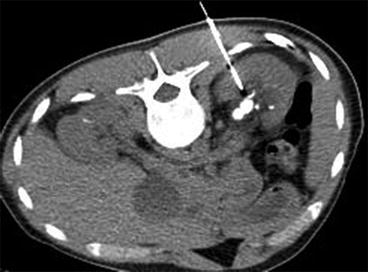

Fig. 7.10
CT-guided supra-11th rib puncture
7.3.4 Radiation Safety
All imaging involving ionized radiation must be conducted according the ALARA principle. ALARA is the acronym for As Low As Reasonable Achievable. The X-ray tube should always be placed under table, and the patient should be placed as close to the image intensifier as possible in order to reduce scattered radiation to the patient, the surgeon and the OR staff. Use of collimation will further reduce scattered radiation and avoid direct exposure of the surgeon and areas of the patient adjacent to the area of interest [1]. All personnel should of course wear lead aprons, and those close to the X-ray source collar guards as well. Regarding the safety distance, the inverse-square law may be applied: radiation dose is reduced by the square of the distance to the X-ray source. This means by doubling or tripling the distance to the source, the dose is reduced by one quarter and one ninth, respectively. In other words, by backing away from the patient during fluoroscopy, the surgeon and the assistants will dramatically reduce radiation exposure. In a study of radiation exposure during ureteroscopy and PNL, average fluoroscopy time was found to be 1.3 and 10.7 min, respectively [31]. The highest scattered radiation was recorded at the lower extremities and the lowest at the level of the eyes. The estimate of maximum scatter radiation exposure to the surgeon for 50 PNL procedures did not exceed 10 mGy, which is below 2 % of the established allowable dose limit for radiation exposure in Great Britain [1, 31]. Nevertheless, medical personnel involved in PNL should be aware of scattered radiation risks and always minimize radiation exposure according to the ALARA principle.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








