Fig. 9.1
(a, b) Percutaneous lymph node biopsy. (a) Axial PET/CT fusion image demonstrating active nodal disease adjacent/enveloping left common iliac artery due to lymphoma. (b) Axial non-contrast CT with patient prone, demonstrating biopsy needle within the left para-aortic/iliac chain nodal disease
Newly developed technologies exist enabling fusion of previously acquired CT images with procedural US images or MR images with procedural CT to facilitate accurate and safe biopsy procedures by optimising tumour localisation, with potential for reduced radiation dose [14, 15]. Functional information from positron emission tomography (PET) can also be fused with anatomical detail from either CT or MRI to facilitate biopsy procedures performed under CT, MRI or US guidance [14, 15].
9.4 Vascular Access
9.4.1 Central Venous Catheters (CVCs)
Indications: Short-term vascular access for medications (e.g. antibiotics), chemotherapy, total parenteral nutrition (TPN) and atraumatic/needleless venous sampling [2].
Results: Image-guided central vascular access has been shown to be more efficient and safer than venous access based on external landmarks [16].
Technique: Involves placement of a catheter with its tip at the atriocaval junction or within the right atrium, usually using a modified Seldinger technique via the internal jugular vein, with a combination of real-time US and fluoroscopic guidance [17, 18].
Complications: Intraprocedural/early complications relate to surrounding tissue injury such as pneumothorax (<1 %), arterial puncture (1–2 %), haematoma or haemorrhage (up to 2 %) and air embolism (up to 1 %). These potential complications are common to other centrally inserted central venous access devices including tunneled haemodialysis catheters and implantable ports, and are less common when using image guidance [2, 18]. Longer-term problems unaffected by technique of insertion include infection (local infection 1–7 %, catheter-related bacteraemia 1–4 %) and thrombosis (up to 28 % of cancer patients) [2, 18]. Reported pulmonary embolus rates vary between 15 and 25 % of those with CVC-related thrombosis [2, 19, 20]. Thrombosis risk is greater in those with multi-lumen catheters [2, 21].
9.4.2 Peripherally Inserted Central Catheters (PICCs)
PICCs are inserted via a peripheral upper arm vein into the central venous system and range from single to triple lumen catheters up to 7 Fr in size [18]. PICCs are designed for intermediate term use up to 2 months and can be used for venous sampling and TPN also. Recent advancements in PICC design allow for high-pressure injections such as in contrast CT studies. Risk of blood stream infection is low at around 2.1 infections per 1,000 catheter days [18].
Tunneled PICCs can also be performed if peripheral venous access is too difficult to achieve. This method involves initial venous puncture into the jugular or subclavian vein before creating a subcutaneous tunnel in the anterior chest wall. This is similar to the insertion method of tunneled haemodialysis and apheresis catheters.
9.4.3 Infusaport
Another device providing long-term central venous access, but with a reservoir (port) that sits in a subcutaneous pouch in the anterior chest, connecting to catheter tubing with tip in the atriocaval junction. The port can be accessed using a non-coring (Huber) needle through the skin [18]. Ports have now been developed that can tolerate high-pressure injections for contrast CT studies. The port insertion procedure is usually performed under light sedation with ample local anaesthetic using ultrasound guidance for initial venous puncture and fluoroscopy for catheter tip placement.
The infection rate is lower than for external catheters, but the consequences of infection are more troublesome, potentially requiring port removal and alternate siting of the device (e.g. contralateral chest or arm) or obtaining alternate access (e.g. PICC). The lowest risk of bloodstream infection amongst the aforementioned central venous access devices at 0.1 infections per 1,000 catheter days [18].
9.4.4 Tunneled Haemodialysis and Apheresis Catheters
This is a larger calibre (up to 14.5 Fr) double lumen central access catheter mainly used for dialysis access in the setting of renal failure or plasmapheresis in the setting of haematological malignancy and stem cell transplant therapy [2, 18]. Insertion is in the same way as a tunneled PICC with majority of access via the jugular vein with a subcutaneous tunnel over the anterior chest wall. The procedure is usually performed with light sedation and ample local anaesthetic. The subclavian vein can also be utilised but has higher rate of associated central venous stenosis or occlusion (up to 50 %) [18]. Translumbar access of the inferior vena cava (IVC) has also been described [18].
9.5 Nutritional Support
Nutrition is an important and ongoing issue for cancer patients, particularly towards the end of life, and especially in patients with head and neck malignancy [22]. Adequate nutrition has been shown to improve therapeutic tolerance and chemotherapy response [22]. Options include TPN that is utilised for those patients without adequately functioning gastrointestinal tracts, or enteral routes for those with functioning bowel [23]. Enteral nutrition has fewer complications than TPN [18]. Enteral feeding tubes can be inserted via an oral/nasal approach or percutaneously into the stomach or small bowel, using fluoroscopic techniques (Fig. 9.2a, b) [1, 18].
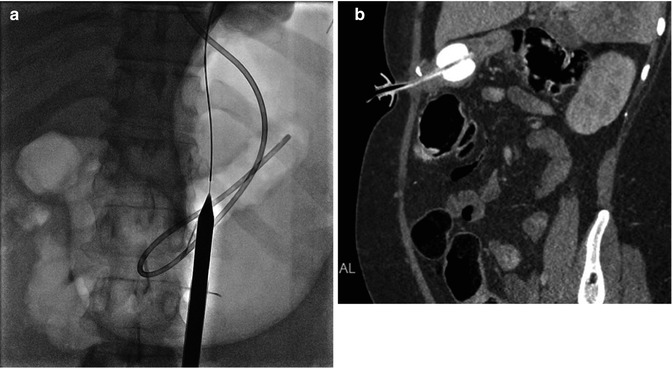

Fig. 9.2
(a, b) Gastrostomy insertion. (a) Demonstrates gastric distension via gas inflation through existing nasogastric tube, with guidewire and percutaneous dilator in place. (b) Oblique Sagittal CT shows satisfactory position of radiologically inserted percutaneous gastrostomy
9.5.1 Oropharyngeal Tubes
Indications: Short-term enteral feeding (or gastric decompression) with tip placement in the stomach or post-pyloric small bowel (jejunum).
9.5.2 Radiologically Inserted Gastrostomy (RIG)
Indications: Long-term or permanent feeding in patients with swallowing difficulties and end stage malignancy, most commonly head and neck cancers, who are unsuitable for percutaneous endoscopic gastrostomy (PEG) insertion due to pharyngeal or oesophageal obstruction [1, 18, 22, 25].
Absolute contraindications: Unsatisfactory anatomy (e.g. overlying transverse colon), uncorrectable coagulopathy and very short life expectancy [18].
Results: With meta-analysis, technical success of RIG placement nears 100 % with low procedure-related mortality (0.3 %) and less complications than other insertion methods [1, 18, 22]. No prospective randomised control trials (RCTs) compare RIG, PEG and surgical gastrostomy insertion, but multiple studies have indicated RIG is superior and is the most cost-effective approach, once endoscopic failures are considered [1, 22, 26, 27].
Complications: Complications are uncommon (5.9 %) but early on include mild discomfort with infusions and infection [2, 18]. Peritonitis can occur from leakage of gastric juices into the peritoneal cavity or incorrect tube placement [18]. If tube dislodgement occurs 2 weeks or more after insertion, then the tract is usually sufficiently matured to enable replacement without need for repuncture or procedural sedation [2, 18]. RIG techniques often incorporate gastropexy with insertion of small metallic stay/anchor sutures to maintain access to the stomach wall in the short term and prevent tube dislodgement [1, 18]. The sutures are usually cut after 1–2 weeks, allowing the anchors to pass into the bowel [1, 18].
9.5.3 Gastrojejunostomy and Jejunostomy Tubes
These tubes can be placed in those patients who have significant gastroesophageal reflux or are at risk of aspiration pneumonitis, noting that gastrostomies do not eliminate aspiration risk and may even increase the risk due to effect on gastric emptying [18, 28]. They may be placed directly into the jejunum or be converted from existing gastrostomy, which is more successful after initial fluoroscopic insertion (93 % success) compared to a primary endoscopic or surgical approach (68 % success) [1, 29]. Complication rates are similar to gastrostomy [2].
9.6 Symptom Relief
9.6.1 Pain
Pain is a common symptom amongst oncology patients and a significant cause of morbidity, particularly in advanced disease [2, 30, 31]. Cancer pain can be multifactorial, acute and chronic and related to the primary disease process or treatment effects, but the most common pain issue in advanced disease is bone and visceral pain [30–32].
In a significant amount of cancer patients (5–14 %), pain is not adequately controlled with medical treatment [30, 33, 34]. Interventional pain management techniques are an additional option in such patients. These procedures incorporate drug delivery to the spinal cord or major nerve plexuses, application of neurolytic agents to neural structures and can provide short-term or permanent pain relief [30, 35]. These procedures can be performed under ultrasound for superficial structures or under CT guidance for deeper targets.
Vertebroplasty as a treatment method for bone pain will be discussed later in this section.
Image guidance for pain interventions has not been demonstrated in the literature to be more effective or safer than without guidance. However, the lack of supportive evidence is unlikely to change practice given the inherent logic that image guidance should help.
9.6.1.1 Central Neural Blockade
Intrathecal opioids have been used in cancer patients for pain control since the late 1970s [30, 35]. Addition of adjuvant drugs such as local anaesthetics and/or steroids to the injectate provides more effective analgesia than single agent injections [34]. Opioid dosing requirements are greater in the epidural space compared with the intrathecal administration, either as single injection or via infusion catheters [34]. When used, image guidance for epidural or intrathecal injections can be with CT or fluoroscopy for thoracic or lumbar region injections, whilst fluoroscopy is more often used for cervical injections. Epidural blocks with steroid and local anaesthetic are usually performed for vertebral cancer-induced bone pain, although evidence is limited [34].
Indications: Pain not adequately covered with oral analgesics or where the side effects of treatment are too inconvenient. Head and neck cancer patients with pain can benefit from cervical epidural steroid injection in particular.
Results: Intrathecal opioids are effective in treatment of cancer pain, and a small survival benefit has also been shown [30, 34].
Complications: The most feared complication is that of infection but fortunately is of low incidence up to 4.6 % superficial infections and 1.2 % deep infections (increased with longer duration of catheter placement) [30, 34]. Other self-limiting complications include dural puncture headache (15 %), epidural haematoma (0.5–0.9 %) and cerebrospinal fluid (CSF) leak [30, 34]. Medication effects also warrant consideration including respiratory depression due to cephalad spread of opioids, weakness and sensory disturbance (from local anaesthetics at higher doses) [34]. Complications of epidural injection of steroid are usually a result of misplacement of the needle (e.g. intrathecal injection, epidural haematoma, vascular injury).
9.6.1.2 Neurolysis
Permanent pain relief with intrathecal injection of alcohol, particularly in advanced disease.
9.6.1.3 Neural Blockade
Coeliac Plexus Block
Located anterior to the coeliac trunk at the L1 vertebral body level and supplying most of the abdominal viscera, the coeliac plexus can be blocked by anterior or posterior approaches, most commonly under CT guidance, using alcohol or phenol injectates, from a posterior approach [30, 34–36].
Indications: Intractable pain caused from abdominal tumours arising from the upper and lower gastrointestinal tract, pancreas, kidney and adrenal origin may benefit from a coeliac plexus ganglion block [30, 31, 34–36].
Superior Hypogastric Plexus Block
Located in the retroperitoneum on both sides of the midline at the L5/S1 disc level close to the bifurcation of common iliac vessels [34].
Indications: Pelvic pain due to gynaecological, colorectal and genitourinary cancers [34].
Ganglion Impar Block
Retroperitoneal in location at the termination of paired vertebral sympathetic chains at the sacrococcygeal junction. CT, fluoroscopic and even ultrasound guidance can be used to facilitate needle placement anterior to the sacrococcygeal junction [38].
Indications: Rectal or perineal pain from genitourinary and colorectal cancers [38].
Lumbar Sympathetic Chain Block
Lumbar sympathetic chain block can be considered for inoperable peripheral vascular disease, less commonly visceral abdominal and pelvic cancer pain (urological) and rectal tenesmus [34].
Stellate Ganglion Block
Stellate ganglion block is used for temporary relief or autonomic pains in the head, neck and upper limbs, either by single or repeated injections [34]. This can be safely performed under US guidance, which is safer than fluoroscopic guidance [39]. Neurolysis is generally not performed due to risk of inadvertent arterial puncture in this location with potential for neural injury or stroke [34, 39].
Splanchnic Nerve Block
Not performed as routinely as the coeliac plexus block, the splanchnic nerve block can be considered in those whom have failed coeliac plexus block [31]. The splanchnic nerves arise from the thoracic sympathetic trunk and join the coeliac ganglion after piercing the diaphragm at T11 and T12 levels [31, 36]. The nerves can be targeted under CT guidance with anterior or posterior approach, or with fluoroscopy for posterior approach [31, 36]. Effective in up to 70 % of patients. Complications include pneumothorax, diarrhoea (self-limiting), diaphragmatic paralysis and cardiac arrhythmias [31, 36].
Complications: Coeliac plexus commonly postural hypotension and diarrhoea which are usually self-limiting and transient [30, 31, 34]. Major complications are rare and usually due to suboptimal placement of phenol injectate. This includes anterior spinal artery spasm causing anterior cord ischaemia with permanent paraplegia (0.15 %) [30, 34]. Aortic dissection, haematoma and retroperitoneal abscess formation have also been reported. There is not sufficient evidence to prove that image guidance is safer than blind techniques for injection, but it is postulated that better visualisation of needle tip and critical adjacent structures (e.g. arteries, organs) should improve accuracy of needle placement with reduced risk of complications due to misplacement [39].
9.6.1.4 Peripheral Nerve Blockade
This has a more limited role in the palliative setting and is technically possible for most neural structures using predominantly US guidance for selective injections. Common targets include the suprascapular, paravertebral, intercostal, lumbar, obturator, sciatic and femoral nerves with blockade performed using a long-acting local anaesthetic (e.g. bupivacaine) with or without corticosteroid. Steroids are particularly effective when the peripheral nerve is involved by the disease process. The risk of peripheral nerve neurolysis is neuritis [34].
9.6.2 Pleural Effusions
Malignant pleural effusion is seen in approximately 50 % of patients with widespread malignancy at some stage during their disease [40, 41]. Associated morbidity and mortality is significant, with 30 days mortality between 29 and 50 %, and mean survival of 3 months [2, 40].
The aetiology is lung cancer or breast cancer in 75 %, but also seen in lymphoma, ovarian cancer and gastric cancer [40, 41]. Dyspnoea is the most common symptom affecting quality of life, also cough and chest pain [2, 40–42].
Treatment is usually palliative, aiming for rapid symptomatic relief (reexpansion of underlying lung), whilst minimising discomfort, inconvenience and disruption of daily activities (Fig. 9.3a, b) [2, 40, 41].
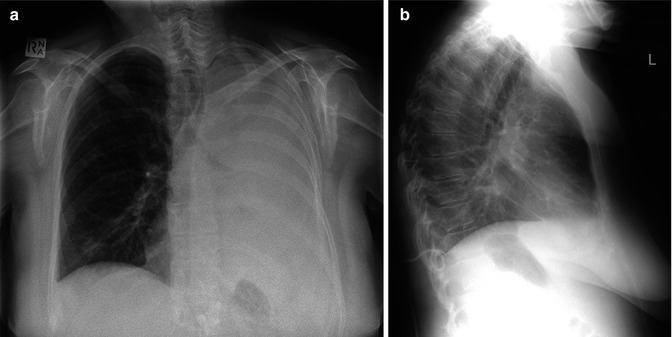

Fig. 9.3
(a, b) Pleural effusion and drainage. (a) Frontal CXR demonstrates large left pleural effusion with left hemithorax whiteout, in patient with left hilar non-small cell lung cancer. (b) Lateral CXR post-insertion of pigtail drain shows tip of drain in the posterior costophrenic recess
US can be utilised for drainage of most pleural effusions and has been shown to save time and improve first puncture success of thoracocentesis, with reduced risk of pneumothorax [40]. CT is also effective for image guidance in more difficult cases [40].
9.6.2.1 Thoracocentesis
Indications: Exudative pleural effusion or loculated effusion. Simple method for diagnosis and immediate short-term symptom relief, but repeated thoracocentesis is not optimal for rapidly reaccumulating malignant effusions [40]. It may be of benefit in those with short survival expectancy or where fluid reaccumulation is slow [40].
Results: Success with US increased up to 88 %, with reduced pneumothorax rate [40]. Malignant pleural effusion will recur within 30 days post-thoracocentesis in nearly all patients [41].
Complications: Uncommon with low rate of pneumothorax (up to 6 %), haemothorax, reexpansion pulmonary oedema, organ laceration, intercostal artery injury and intercostal neuralgia [40]. Approximately half of patients with pneumothorax require formal drainage tube [40]. The bleeding risk is reduced by correcting coagulation parameters prior to the procedure and by ensuring needle placement above the rib margin to avoid the neurovascular bundle [10, 40, 41]. Risks of the procedure increase with repeated thoracocentesis [41].
9.6.2.2 Nontunneled and Tunneled Drainage Catheters
Pigtail drainage tube insertion is commonly performed with US guidance using a modified Seldinger technique following dilatation of the tract, although some direct stick catheters are available [1, 41]. Tunneled catheters are inserted via a similar technique, with the proximal catheter tunneled in subcutaneous tissues to enable longer-term management of effusion due to reduced infection and dislodgement rates [40, 41, 43].
Indications: Long-term management of malignant pleural effusion enabling outpatient management, best with tunneled catheter [40, 41]. Tunneled drains can still be effective after failed formal pleurodesis [40].
Results: Symptomatic relief and control of malignant effusion occurs in 90–95 % of patients with tunneled catheter technique [40]. Spontaneous pleurodesis occurs in approximately 40 %, mostly within 1 month of the tube insertion [40, 41]. Tunneled catheters are safe, cost-effective and enable outpatient management of effusion [40, 43].
Complications: Most common is fluid loculation (8 %), with incomplete drainage [41]. Less commonly empyema, cellulitis, tube dislodgement, bleeding, tumour seeding and extrapleural migration of the catheter [40, 43]. Long-term non-tunneled catheter placement may eventually lead to empyema [1]. Tunneled catheters have the advantage of reduced risk for dislodgement and low risk of complications including tunnel or pleural infections or pericatheter leakage, due to development of a fibrous cuff around proximal portion of catheter, and a one-way valve at proximal hub of the catheter [40].
9.6.2.3 Pleurodesis
Pleurodesis obliterates the pleural space to prevent pleural effusion from reaccumulation [1, 40, 41]. Approximately two thirds of patients with malignant pleural effusion fail to respond to thoracocentesis or drainage catheters, without pleurodesis [40].
Chest tube insertion with pleurodesis and thoracoscopy can require hospitalisation for up to 1 week, which is neither desirable nor cost-effective as a palliative management in patients with a short life expectancy [40]. Intrapleural instillation of sclerosant material can be used to facilitate pleurodesis, and this can be performed as an outpatient, with small drainage catheter pleurodesis as effective as traditional large bore catheter use [2, 40, 41, 44, 45]. The procedure can be undertaken after one or two consecutive drainages of fluid [40]. Sclerosing agents include talc, tetracycline, doxycycline and bleomycin, with talc being the most commonly used [1, 41, 44, 46]. The chest tube can be removed when drainage is less than 50–100 ml/day, although protocols vary by institution [1, 18, 40].
Indications: Recurrent malignant pleural effusion.
Results: Recurrence rates of effusion can be as low as 10 % using talc slurry pleurodesis [1].
Complications: In addition to complications of drain insertion, minimal discomfort and mild fever are common side effects, with talc pneumonitis and respiratory failure less commonly observed [40].
9.6.3 Ascites
Abnormal volume of fluid in the peritoneal cavity as a result of cancer is termed malignant ascites and is a common presentation of malignancy in up to 50 % [1, 47]. Common underlying neoplasms include breast, ovarian, gastric, pancreas and colon cancer, with up to 20 % of unknown primary [1, 41, 48]. Associated life expectancy is short, less than 4 months, with breast and ovarian cancer as exceptions where survival is usually more prolonged [1, 49].
With the exception of those awaiting liver transplant, treatment of recurrent ascites is palliative [1]. Temporary relief of malignant ascites with either blind- or US-guided paracentesis is effective in reducing pain, shortness of breath, anorexia, nausea and improving mobility, although swift reaccumulation of fluid is likely (Fig. 9.4) [1, 41].
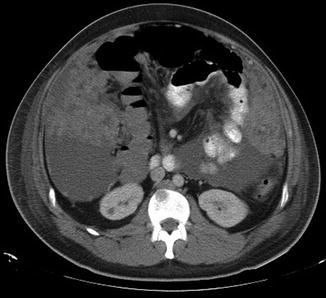

Fig. 9.4
Peritoneal carcinomatosis. Axial CT abdomen demonstrating large volume ascites, omental caking and peritoneal disease in a patient with melanoma
Repeated paracentesis can cause significant morbidity through bleeding, organ injury or infection, dehydration and malnutrition, although the overall complication rate is low (around 1 %) [1, 41].
9.6.3.1 Nontunneled and Tunneled Catheters
Single drainage of ascites with nontunneled catheters is effective but if left in place long term are prone to complications such as infection (35 %), accidental removal, leakage (20 %) and occlusion (30 %) [1, 41]. As a result, unless the patient has a short life expectancy, tunneled catheters are preferred for long-term management of malignant ascites, as they have reduced infection risk [1, 41].
Indications: Malignant ascites.
Results: US-guided tunneled catheters have similar complication rates and patient satisfaction when compared to large volume paracentesis, although the reduced need for hospital visits to undergo paracentesis is an obvious advantage of a tunneled drain [41].
Complications: Peritonitis, leakage and loculation are all uncommon [41].
9.6.4 Collections
Whilst generalised accumulation of fluid in the abdomen can be a cause for presentation or deterioration in a patient’s condition, so too can complications of underlying disease (e.g. cystic liver metastases causing capsular distension and pain, liver abscess in patients with hepatopancreatobiliary malignancy) or treatment complications, such as intra-abdominal or pelvic collections in the setting of debulking surgery or targeted invasive organ therapies (e.g. liver abscess post-ablation or chemoembolisation) [7, 18, 50–53].
Whilst diagnostic aspirations can be performed under local anaesthetic with small calibre needles, symptomatic benefit usually requires percutaneous 6–8 Fr drain insertion (10 Fr or larger for more complex collections) for several days [7]. As with drainage of ascites, collection drainage can be performed percutaneously under US or CT guidance using a modified Seldinger technique. US is suitable for superficial collections or when angled access is required and allows real-time guidance, whereas CT fluoroscopy is safer for deeper collections or those containing gas and gives better anatomical detail [7, 18].
9.6.4.1 Abdominal Fluid Collections and Abscess
Common locations include hepatic, perihepatic (subphrenic and subhepatic), gallbladder bed, splenic bed, lesser sac, paracolic and the retroperitoneum [7].
Indications: Collection greater than 4 cm, symptomatic [7]. Collections <4 cm in diameter may well respond to conservative treatment, although aspiration when symptomatic can be beneficial [7].
Results: Percutaneous drainage is a safe, effective alternative to surgery with low complication rate (5 %), morbidity and likely a shorter hospital stay [7]. Multiple or multi-loculated collections are more challenging, and although there are high success rates for drainage in the noncancer setting up to 90 %, outcomes are not as impressive in infected tumour drainage, with higher rates of secondary or permanent drainage being required [7, 54, 55]. This relates to infected tumour tending to be a preterminal entity, with complete drainage unlikely unless the underlying tumour resolves, and these patients are often unfit for surgery to definitively treat the tumour [54]. Those with underlying hepatopancreatobiliary malignancy have poorer outcomes of drainage than in other malignancies [50].
9.6.4.2 Pelvic Fluid Collections
Indications: Postoperative collections or lymphoceles, diverticular abscess, cystic tumour or infected tumour are common indications for drainage in patients with malignancy [7, 18]. Whilst infected collections can be symptomatic due to sepsis, other presentations can relate to mass effect of the collection, such as intractable pain and bowel obstruction [7, 18, 56].
Contraindications: Lack of safe access route and irreversible coagulopathy.
Modality for drainage: Collections superficial to the abdominal muscles can be drained with US guidance. Deeper collections may require CT to identify collection as separate from the bowel [7]. Posterior transgluteal approach via the greater sciatic foramen can be used in deep pelvic abscesses [7, 56]. Alternatively, transrectal (if adjacent rectum) or transvaginal (if in contact with vagina) drainage may be more feasible or even transperineal [7, 18, 56].
Results: Successful in up to 90 % in the noncancer setting and best if the collection is postsurgical, with slightly poorer outcomes associated with infected tumour or fistula [7, 56].
9.6.5 Biliary Obstruction
9.6.5.1 Biliary Drainage and Stent
Obstructive jaundice can cause pruritus, cholangitis, sepsis, hepatic dysfunction and malnutrition and is associated with short life expectancy if untreated [1]. Endoscopic treatment of distal biliary obstruction due to pancreatic neoplasm is an effective treatment for the majority of patients [2, 57]. The role of percutaneous treatment is for effective primary or palliative management in those who fail endoscopic intervention, are unfit for endoscopy, or in those with proximal duct obstruction (due to hilar lymphadenopathy, hilar metastatic liver disease) [2, 57]. The efficacy of nonsurgical palliative treatments varies according to the location of obstruction, the technique and extent of decompression and the biliary stent prosthesis used, but biliary stents improve quality of life [1, 57].
Percutaneous drainage can be performed using a combination of US and fluoroscopic guidance [7]. Once biliary access is established, it may be possible to traverse the obstruction/stricture (using catheters and wires) to enable insertion of a stent or placement of an internal-external biliary drain as a temporising measure prior to stent insertion (Figs. 9.5a–d and 9.6a–c) [7, 57]. Percutaneous stent placement is less preferred than endoscopic metal stent placement due to theoretically more bleeding and pain complications, but this has not been confirmed in RCTs [1]. If the obstruction cannot be passed, then an external biliary drain is the only option, and this will likely be permanent, requiring regular drain exchanges to reduce infection risk and maintain patency [1, 7, 57].
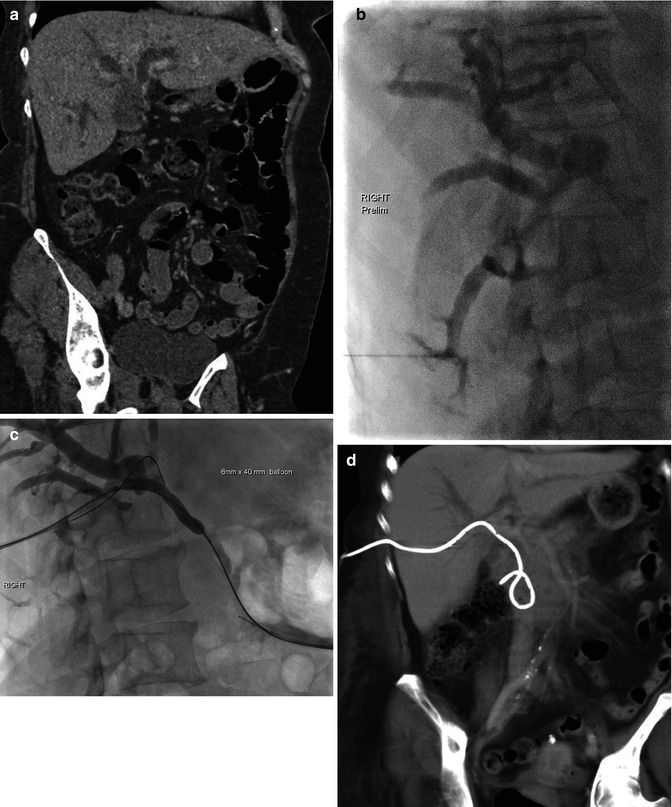
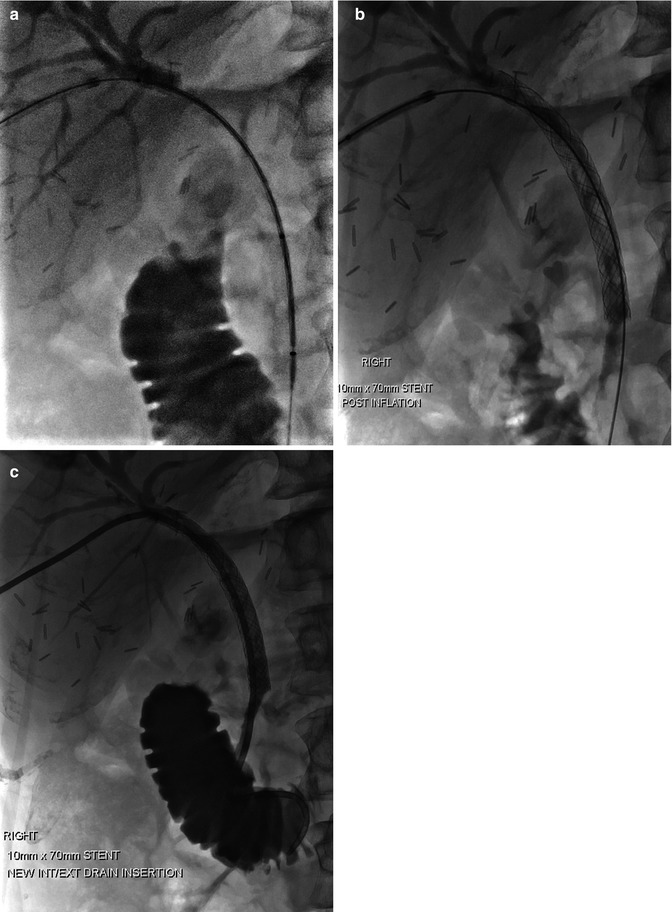

Fig. 9.5
(a–d) Biliary drain. (a) Coronal portal venous phase CT abdomen demonstrating marked intrahepatic biliary dilatation due to gallbladder carcinoma infiltrating ducts at the hilum. (b) Percutaneous cholangiogram with contrast identifying dilated intrahepatic ducts, but no drainage into the common bile duct (CBD). (c) Balloon dilatation of the tract in the region of the hilum and CBD having crossed the stenosis with wire placement across CBD and into the duodenum. (d) Demonstration of drain position on coronal portal venous phase CT abdomen with some associated reduction in degree of biliary dilatation

Fig. 9.6
(a–c) Biliary stent. (a) Procedural x-ray with wire and catheter across hilar and CBD stricture following percutaneous cholangiogram. (b) Post deployment and balloon dilatation of the stent. (c) Final stent position with insertion of additional internal-external biliary drain as a precaution to maintain access, in case of early stent blockage
Another topic of debate is that of metal versus plastic stents. Self-expanding metal stents have been shown to have better patency rates than plastic drains and plastic stents [1]. An advantage of plastic drains is that the patient is aware of blockage as soon as it happens due to bile leakage through the drainage bag or around the drain catheter [1]. Post-procedure pain associated with percutaneous drainage can usually be controlled with oral analgesia [1].
Less commonly, combined procedures with an endoscopist can be performed with side by side insertion of a duodenal or hepaticoduodenal stent. A problem with stent insertion is that they can block and need redilatation with a balloon catheter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






