Gland
Secretion
Function
Principle cells [3] (epididymus)
Glycerophosphocholine
Inhibits motility and premature capacitation
Seminal vesicles [3]
Viscous fluid rich in fructose and glucose, several proteins, including fibrinogen and prostaglandins
Substrates for anaerobic metabolism; fibrinogen initiates postejaculatory coagulation; prostaglandins contribute to make the female environment more hospitable
Prostate [3]
Alkaline secretion containing calcium, albumin, proteolytic enzymes, acid phosphatase, fibrinogenase, profibrinolysine, zinc, citric acid, and lipids
Neutralisation; fibrinogenase for postejaculatory coagulation; profibrinolysine for liquefaction; zinc stabilizes the DNA; citrate contributes to the pH buffering capacity
Bulbourethral glands [3]
Clear fluid
Lubrication for easy expulsion of the semen; neutralization; aids the mobility of spermatozoa by creating less viscous channels
Epididymis [7]
Lymphocytes and macrophages
Reactive oxygen species [55] (ROS) which has beneficial functions at physiological levels
Prostate and seminal vesicles
Granulocytes [7]
Following spermatogenesis in the testes, the sperm are stored and they undergo further maturation in the epididymis, where the principle cells secrete glycerophosphocholine, inhibiting both motility and premature capacitation [3]. In this metabolically-suppressed state, spermatozoa can survive in the male reproductive tract for many weeks.
A normal, mature spermatozoon consists of a head, midpiece, and flagellum. The head, containing a haploid set of DNA material, is covered with a cap-like structure known as the acrosome. The midpiece houses the mitochondria, which provide ATP via oxidative phosphorylation, and the flagellum is used to propel the spermatozoa and contains glycolytic enzymes for ATP production by glycolysis.
During ejaculation, as the spermatozoa pass through the ejaculatory ducts, the accessory sex glands contribute various secretions that ultimately constitute the bulk of the seminal plasma. The seminal plasma consists of several components intended to sustain and promote survival of the spermatozoa. Key features of semen include its high buffering capacity, maintaining its pH to almost neutral in acidic environments, as well as a substantially high osmolarity in comparison to blood plasma (hypertonic) [4], a feature which is influenced greatly by the concentrations of sugars, ionic salts, and other organic compounds [5] and which has been noted to increase significantly with age [6]. Seminal plasma contains more than 50 different chemical compounds, each of which assists sperm survival and function in the female reproductive tract.
Ejaculation comprises of two phases: during the first phase, the emission stage, the spermatozoa are transported from the epididymis to the urethra via smooth muscle contraction; while during the second phase, the true ejaculatory phase, seminal plasma is moved through the urethra and, along with the sperm, expelled from the body as semen [7].
The seminal vesicles are the first to add their secretion to the seminal plasma, producing a viscous fluid rich in nutrients such as fructose (274 mg/100 ml) [8] and glucose, at an average concentration of (102 mg/100 ml) [4], as well as several proteins, including fibrinogen and prostaglandins [3]. The sugars serve as substrates for anaerobic metabolism (fructolysis and glycolysis), which the spermatozoa will utilize as soon as they gain motility. Fibrinogen initiates postejaculatory coagulation of the seminal plasma, while the prostaglandins help to make the female environment more hospitable to the spermatozoa in terms of pH-buffering [3].
The prostate gland contributes an alkaline secretion containing calcium, albumin, proteolytic enzymes, acid phosphatase, fibrinogenase, profibrinolysine, zinc, citric acid, and lipids [3]. Since spermatozoa are extremely susceptible to an acidic environment, the most important function of the prostatic secretion is neutralisation of (i) the vas deference, which is acidic due to metabolic waste such as increased CO2 and lactic acid produced by the spermatozoa, and (ii) the vagina, which is acidic due to the natural bacteria it contains. The fibrinogenase assists in postejaculatory coagulation, and the profibrinolysine leads to liquefaction [3]. Zinc helps to stabilize the DNA-containing chromatin [9]; citrate contributes to the pH buffering capacity and is also believed to be the major regulator of ionized calcium levels in the seminal plasma [10, 11]. The calcium concentration is a key factor in sperm motility, metabolism, acrosome reaction, and fertilization [12].
Additional cells that may be found in seminal plasma are lymphocytes and macrophages, which originate mainly from the epididymis, as well as granulocytes that are derived from both the prostate and seminal vesicles [13]. Activated granulocytes release reactive oxygen species (ROS), which have beneficial functions at physiological levels, but can become detrimental when elevated, in which case it disrupts the spermatozoa’s membranes and compromises DNA integrity [13].
Finally, the bulbourethral glands secrete a clear fluid into the lumen of the urethra, providing lubrication for easy expulsion of the semen [3], as well as buffers to assist in neutralization of the acidic vaginal pH [14]. This mucus furthermore aids the mobility of spermatozoa in both the vagina and cervix as it creates channels that are less viscous through which the spermatozoa can swim.
The World Health Organization describes normal human semen as having a volume of at least 1.5 ml, pH of around 7.2, sperm concentration greater than 15 × 106 spermatozoa/ml, and total motility of at least 40 % with forward progression of 32 % within 60 min of ejaculation [15].
Function of Spermatozoa in the Female Reproductive Tract
Introduction
Despite being motile once inside the female reproductive tract, spermatozoa are not yet capable of fertilizing an oocyte at this stage. Spermatozoa require a species-dependant amount of time to travel from the site of deposition, in the vagina, to the site of fertilization, in the fallopian tubes [16], while simultaneously acquiring fertilizing ability by undergoing a series of complex changes along the way, collectively referred to as capacitation [16].
Capacitation
The process of capacitation has been defined as a “functional maturation of the spermatozoon” [17]. Capacitation involves considerable alterations to the surface of the plasma membrane, with various molecules being removed, rearranged, or revealed. These changes are facilitated by the removal of cholesterol and glycoproteins, resulting in a more fluid plasma membrane with an increased permeability to Ca2 + 16. The subsequent upsurge in Ca2 + leads to increased intracellular cAMP levels and, consequently, increased motility [3].
The uterus aids in the steps of capacitation by secreting sterol binding albumin, lipoproteins, and glycosidases such as heparin [18]. Sterol binding albumin promotes cholesterol efflux from the sperm plasma membrane [19], while heparin [20] and lipoproteins [21] have been shown to accelerate the initiation of both capacitation and the acrosome reaction in vitro. Extracellular Ca2 + plays an important role as a signalling molecule during capacitation and the acrosome reaction, and is a prerequisite for both processes [16]. The acrosomal area also undergoes changes during capacitation in preparation for a possible acrosome reaction closer to fertilization.
As a result of the increased intracellular Ca2 + levels mentioned before, and by the time the fallopian tubes are reached, the spermatozoa become hyperactivated, indicating the successful completion of the capacitation process.
Hyperactivation
Hyperactivation is a motility pattern usually occurring in vivo in the fallopian tubes [22], and is characterized by increased amplitude and asymmetrical flagellar beating [23] together with increased amplitude of lateral head displacement (ALH) [24]. Hyperactivation is triggered by a rise in intracellular flagellar Ca2 + and requires an increase in both pH and ATP production [23]. Since, hyperactivation is normally only displayed at the site of fertilization, it may therefore be modulated by chemotactic signals to direct and turn spermatozoa toward the oocyte [23], while the increased sideways displacement of the head increases the spermatozoon’s chances of encountering the egg [22]. Furthermore, hyperactivation enhances the ability of the spermatozoa to traverse the viscous fluids in the fallopian tubes, affording increased flexibility and facilitating the penetration of the spermatozoon through the cumulus complex and zona pellucida [22].
Acrosome Reaction
The oocyte is surrounded by various external structures; the outermost is the cumulus oophorus, a gel-like hyaluronic acid that the spermatozoa encounters initially and needs to penetrate first. Inside of the cumulus is the zona pellucida, to which sperm must bind. This acts as the final mechanical barrier for spermatozoa before fertilization. In order for the spermatozoa to fuse with the oolemma, it must bind to the zona pellucida, reorientate, and finally, penetrate the oocyte [25]. The zona pellucida consists predominantly of glycoproteins, among which ZP1, ZP2, ZP3, and ZP4 [26] are highly species-specific and complimentary to glycoproteins on the surface of the head of the spermatozoa. The zona glycoproteins ZP1, ZP3, and ZP4 are primarily responsible for the tight binding of capacitated, acrosome-intact spermatozoa and initiating a cascade of cellular interactions that culminate in fertilization. In vivo, the acrosome reaction is initiated when the spermatozoon comes into contact with or in very close proximity to the oocyte’s cumulus layer. The purpose of the acrosome reaction is the release of the enzymes (hyaluronidase and acrosin [27]) contained inside the acrosomal cap, which is responsible for digestion of the zona pellucida and oolemma in order to allow the spermatozoon to penetrate the oocyte. The enzymes that are released digest the cumulus cells surrounding the oocyte, exposing the oocyte to the acrosin attached to the inner membrane of the sperm. Acrosin digests the zona pellucida and membrane of the oocyte. Progesterone, secreted by the cumulus cells, is an important cofactor for the initiation of this process [27], as is the increase in calcium permeability due to successful capacitation [28].
Upon completion of the acrosome reaction, the inner membrane of the spermatozoa subsequently fuses with the oolemma and the contents of the sperm head enter the oocyte during a process termed penetration [29].
Fertilization
Upon penetration, the oocyte becomes activated. It completes its secondary meiotic division and the two haploid nuclei—paternal and maternal—fuse to form a diploid zygote. In order to prevent polyspermy and minimize the possibility of producing a triploid zygote, several changes to the oocytes’ membranes render them impenetrable shortly after the first sperm enters the egg through a process called the zona reaction [29].
Metabolism
Being independent living cells, spermatozoa must sustain themselves and support their functions and motility for an extended period of time under varying conditions [30], and as they are not directly attached to the female body or linked to the bloodstream, they must survive on very limited resources. Spermatozoa are maintained at a low energy consumption state during epididymal storage, conserving energy and favoring long-term cell survival [3]. Despite being very small cells, spermatozoa require a substantial amount of energy in the form of ATP in order to support cellular processes and functions such as protein phosphorylation and motility [30]. The preferred metabolic pathway for ATP production varies greatly between species.
In humans, immature spermatids seem to favor the substrates lactate and pyruvate, indicating that oxidative phosphorylation is the main energy source during early spermatogenesis. However, as spermatozoa undergo maturation in the epididymis, they expand their energy production ability to furthermore utilize glycolysis as an alternative source [30].
While the acrosome reaction utilizes lactate and pyruvate for ATP production by oxidative phosphorylation, gamete fusion requires glucose to produce NADPH by the pentose pathway. Normal sperm motility appears to be sustained by relatively low ATP levels, but increased ATP levels are required for tyrosine phosphorylation linked to hyperactivation. Thus, each individual process and event requires a different substrate and metabolic pathway [30], although it is important to note that utilization of these two metabolic pathways is not mutually exclusive.
Glycolysis appears to be indispensable to the spermatozoa of all species and has been shown to compensate for a lack of oxidative phosphorylation, thereby leading to the recovery of most sperm functions. Spermatogenic glycolytic enzymes may be more flexible in the use of substrates, and able to adapt to unexpected conditions in the female reproductive tract [30].
Ultimately, it appears that the metabolic pathway utilized in vivo is greatly influenced by the conditions of the female reproductive tract [31], which, in turn, may be influenced by a variety of factors, not the least of which is the menstrual cycle.
Motility/Swimming Patterns
After sperm have been in the epididymis for 18–24 h, they develop the capability of motility, even though several inhibitory proteins in the epididymal fluid still prevent final motility until after ejaculation. Smooth muscle contractions of the female reproductive tract, ciliary beats, fluid currents, and flagellar activity of sperm are primary mechanisms of sperm movement and transport [32]. Sperm can live for many weeks in the suppressed state in the male reproductive tracts, but the life expectancy of ejaculated sperm in the female genital tract is only about 5 days [3].
Normal motile, fertile spermatozoa are capable of flagellated movement at velocities of up to104 mm/min. The motility of sperm is significantly enhanced in a neutral and slightly alkaline medium, as it exists in the ejaculated seminal plasma, but it is greatly suppressed in even a slightly acidic medium. The activity of spermatozoa accelerates markedly with increased temperature, but so does the rate of metabolism, leading to a reduction in the lifespan of the spermatozoa.
Activated motility as seen in freshly ejaculated spermatozoa refers to the low amplitude symmetric waves propagating along the length of the flagellum and resulting in linear propulsion of the sperm cell [33]. Hyperactivated motility, as seen at the site of fertilization occurs when the flagellar movement becomes asymmetrical with higher amplitude, resulting in highly curved trajectories [34].
Survival of Spermatozoa in the Female Reproductive Tract
Introduction
The passage of sperm through the female reproductive tract is regulated to maximize the chance of fertilization and ensure that morphologically and functionally normal spermatozoa will be the ones to succeed. A single spermatozoon is about 60 μm long, and needs to swim a distance of approximately 20 cm to reach the oocyte. Millions of spermatozoa are produced by the male as a first attempt to increase the chance of successful fertilization, as many spermatozoa die along the way. Spermatozoa must survive the journey independently and without the benefit of reparative mechanisms available as is the case with somatic cells [35], while being subjected to various physical and chemical stressors (refer to Fig. 6.1). In humans, it has been observed that sperm are still viable and fertile in the female up to 5 days after intercourse.
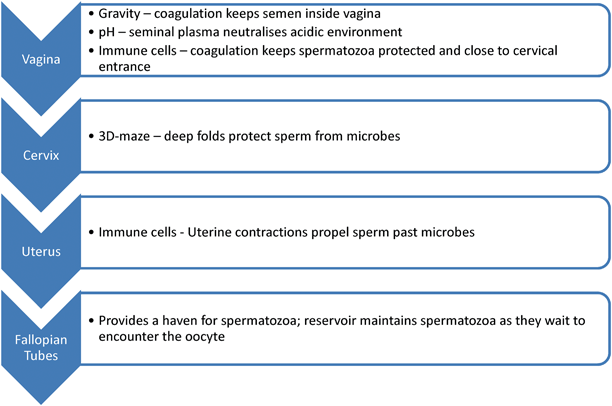

Fig. 6.1
Anatomical pathway, challenges and surviving mechanisms of spermatozoa in the female reproductive tract
The remainder of this chapter will be dedicated to the obstacles spermatozoa face in the female reproductive tract, and, more importantly, the mechanisms that exist to aid in the survival of the spermatozoa in this hostile environment.
Vagina
During intercourse, semen deposition is usually confined to the anterior vaginal region, near the cervical os. Spermatozoa deposited in the vagina face two major complications: gravity and a sudden acidic environment. Upon ejaculation, the semen immediately coagulates in the vagina and liquefies after 30–60 min. This initial clotting of the semen inside the vagina not only prevents sperm loss and leaking due to gravity, but it furthermore provides the spermatozoa with a pH buffered and substrate rich milieu in the interim. During the sequential liquefaction process mucus molecules present in the seminal plasma arrange themselves to form channels that help guide the motile spermatozoa out of the plasma coagulum and toward the opening of the cervix. This process serves as the first selection step toward a viable sperm fraction, as immature/immotile sperm will not be able to migrate efficiently from the seminal plasma [35].
Being exposed to the external environment, the vagina is susceptible to infections, and is therefore well equipped with antimicrobial defenses, which, in addition to the acidic pH, includes high incidence of immune cells [35]. However, due to the prostaglandins’ immune-suppressing effects and pH buffering of the seminal plasma, spermatozoa can survive in the vagina for 24–48 h before they are removed by the female immune cells [3].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








