Hypospadias results from abnormal development of the penis that leaves the urethral meatus proximal to its normal glanular position anywhere along the penile shaft, scrotum, or perineum. A spectrum of abnormalities, including ventral curvature of the penis (chordee), a hooded incomplete prepuce, and an abortive corpora spongiosum, are commonly associated with hypospadias. Advances in understanding of the causes of hypospadias and current approaches to the correction of hypospadias to provide a cosmetically and functionally satisfactory repair are the focus of this article.
Hypospadias results from abnormal development of the penis that leaves the urethral meatus proximal to its normal glanular position anywhere along the penile shaft, scrotum, or perineum ( Fig. 1 ). A spectrum of abnormalities, including ventral curvature of the penis (chordee), a hooded incomplete prepuce, and an abortive corpus spongiosum, are commonly associated with hypospadias.
Hypospadiology is a term coined by John W. Duckett, Jr., the former chief of the Division of Urology at the Children’s Hospital of Philadelphia (CHOP) and a pioneer in hypospadias repairs. Hypospadiology encompasses a continuously evolving and expanding discipline. Although modern experiments have only recently begun to yield a deeper understanding of the genetic, hormonal, and environmental basis of hypospadias, the quest for a surgical procedure that consistently results in a straight penis with a normally placed glanular meatus has occupied surgeons for more than two centuries. Advances in understanding of the causes of hypospadias and current approaches to the correction of hypospadias to provide a cosmetically and functionally satisfactory repair are the focus of this article.
Etiology
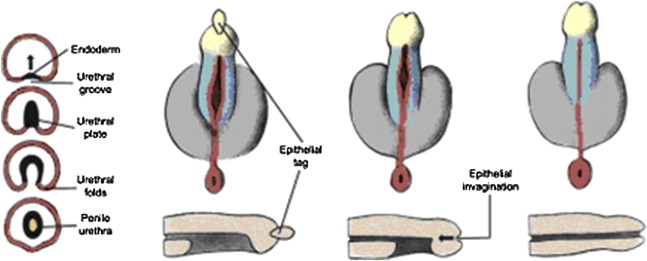
In normal development, the urogenital folds fuse to form the penile urethra. A small region of the distal urethra in the glans is formed by the invagination of a surface epithelial tag ( Fig. 2 ). Hypospadias results from partial or complete failure of urethral folds to form throughout their normal length or a failure of the folds to close distally if they have formed. The extent of the closure determines the position of the urethral orifice.
A unifying etiology for hypospadias remains elusive and is likely multifactorial. The hypospadiac anatomy appears consistent with incomplete embryologic development due to (1) abnormal androgen production by the fetal testis, (2) limited androgen sensitivity in target tissues of the developing genitalia, or (3) premature cessation of androgenic stimulation due to early atrophy of the Leydig cells of the testes.
Endocrine Factors
Several investigators have suggested that hypospadias represents a mild disorder of sex development (DSD). It could represent one end of a spectrum, the other of which is a completely feminized male. Hypospadias may result from an endocrinopathy in which there is a disruption in the synthetic biopathway of androgens. A qualitative androgen receptor (AR) abnormality or defects at a postreceptor level may explain the defect in some of boys with hypospadias. For example, the blunted response to hCG injections seen in many boys with hypospadias may suggest a mutation in the luteinizing hormone receptor in the testis or perhaps an increase in receptor numbers as a consequence of the previous stimulation. In a study by Allen and Griffin of 15 boys (< 4 years of age) with severe hypospadias, 11 boys were diagnosed with a total of 6 distinct endocrine-related abnormalities. The most consistent finding was a subnormal testosterone response to hCG stimulation in 7 boys. The investigators postulated that their findings may represent a delay in the maturation of the hypothalamic-pituitary-testicular axis.
Genetic Factors
Hypospadias is believed to have a complex genetic background with gene expression acting in concert with environmental factors. The familial rate of hypospadias is approximately 7%, reflecting a nonfamilial, sporadic finding in most cases. Recent studies have suggested a role for müllerian inhibiting substance (MIS) in the etiology of hypospadias. There is an inverse relationship between MIS and testosterone and this may be related to the MIS inhibition of cytochrome P450c17 (CYP17), the enzyme that catalyzes the committed step in testosterone synthesis. MIS may directly inhibit testosterone production by suppressing the CYP17 gene. Abnormalities of other genes, such as fibroblast growth factor 10, have also been shown to result in hypospadias.
Normal sexual differentiation depends on testosterone and its metabolites as well as functional ARs. Despite a correlation of certain clear defects in the androgen metabolism pathway and hypospadias, as in the 5α-reductase defect (mutation in SRD5A2 gene on chromosome 2), such associations have been limited to few cases—underlining the importance of seeking other genetic explanations for this congenital defect.
Environmental Factors
Some experts believe that the incidence of hypospadias is on the rise worldwide. One possible explanation is environmental contamination. It is well known that insecticides, pharmaceuticals, and plant estrogens contain estrogenic ingredients and that metal cans used in the canned food industry are coated internally with plastics known to contain estrogenic substances. These substances are ultimately present in the fresh and seawater in trace amounts that are bioaccumulated and concentrated in higher organisms of the food chain. For this reason, predators at the top of the food chain, such as large fish, birds, sea mammals, and humans, accumulate high levels of estrogenic environmental contaminants. Thus, humans and wild animals are constantly exposed to estrogenic compounds known to disrupt reproduction—the so-called endocrine disrupters. Antiandrogens interfere with testosterone function in many ways, including a conformational change within the AR, increasing AR degradation, or blocking the release of heat shock proteins from AR. Additionally, environmental factors coupled with maternal stress may increase the risk of hypospadias, as has been shown in an animal model.
Maternal Factors
In 1967, Goldman and Bongiovanni suggested a role for maternal progestin exposure in the development of hypospadias. These researchers produced hypospadias in male rats by experimentally inducing congenital adrenal hyperplasia. A disturbance in the maternal-fetal hormonal milieu as a causative factor in humans was substantiated when male offspring conceived by in vitro fertilization requiring progestin therapy had a markedly increased incidence of hypospadias. Fredell and colleagues associated low birth weight with hypospadias in discordant monozygotic twins. Recent evidence suggests that a diet lacking in meat and fish results in a more than 4-fold increased risk of hypospadias. Advanced maternal age may also predispose male infants to more severe forms of hypospadias.
Future Areas of Research
Studies that elucidate the role of cellular signals other than testosterone and dihydrotestosterone in normal phallic development and hypospadias, endocrine disrupters, and mesenchymal-epithelial interaction may hold the key to explaining the etiology of hypospadias. Research in the areas of homeobox (Hox) gene may also open new avenues toward increased understanding of the cause of hypospadias.
Epidemiology
The incidence of hypospadias is rising and varies geographically. Prevalence ranges from 0.26 per 1000 births (male and female births) in Mexico to 2.11 in Hungary and 2.6 per 1000 live births in Scandinavia. A recent study found the rate of hypospadias in a two-year prospective study to be 38 per 10,000 live births in Netherlands, a number six times higher than previously recorded. Sweet and colleagues reported a much lower incidence in Sweden of 1 in 1250 live male births.
In 1997, 2 independent surveillance systems in United States, the nationwide Birth Defects Monitoring Program (BDMP) and the Metropolitan Atlanta Congenital Defects Program (MACDP), reported a nearly doubling of rate of hypospadias when compared with immediately preceding decades. The incidence of all types of hypospadias increased from 20.2 to 39.7 per 10,000 live male births during the period from 1970–1993 (ie, 1 in every 250 live male births was a boy with hypospadias [measured by BDMP]). MACDP reported a rise in severe hypospadias rate of between 3-fold and 5-fold. These rising trends, however, may reflect earlier diagnosis or an increase in reporting to registries of congenital defects. The increased reporting of more proximal than distal hypospadias cases, however, refutes the argument that these findings represent more frequent reporting of minor cases.
Recent studies have linked the rising rate of hypospadias in boys born prematurely and small for gestational age, boys with low birth weight, and boys born to mothers over 35 years of age. Roberts and Lloyd noted an 8.5-fold increase in hypospadias in one of monozygotic male twins compared with singleton live male births. This may suggest a discrepancy in the supply of hCG to the fetus where a single placenta is unable to meet the requirements of two developing male fetuses.
Epidemiology
The incidence of hypospadias is rising and varies geographically. Prevalence ranges from 0.26 per 1000 births (male and female births) in Mexico to 2.11 in Hungary and 2.6 per 1000 live births in Scandinavia. A recent study found the rate of hypospadias in a two-year prospective study to be 38 per 10,000 live births in Netherlands, a number six times higher than previously recorded. Sweet and colleagues reported a much lower incidence in Sweden of 1 in 1250 live male births.
In 1997, 2 independent surveillance systems in United States, the nationwide Birth Defects Monitoring Program (BDMP) and the Metropolitan Atlanta Congenital Defects Program (MACDP), reported a nearly doubling of rate of hypospadias when compared with immediately preceding decades. The incidence of all types of hypospadias increased from 20.2 to 39.7 per 10,000 live male births during the period from 1970–1993 (ie, 1 in every 250 live male births was a boy with hypospadias [measured by BDMP]). MACDP reported a rise in severe hypospadias rate of between 3-fold and 5-fold. These rising trends, however, may reflect earlier diagnosis or an increase in reporting to registries of congenital defects. The increased reporting of more proximal than distal hypospadias cases, however, refutes the argument that these findings represent more frequent reporting of minor cases.
Recent studies have linked the rising rate of hypospadias in boys born prematurely and small for gestational age, boys with low birth weight, and boys born to mothers over 35 years of age. Roberts and Lloyd noted an 8.5-fold increase in hypospadias in one of monozygotic male twins compared with singleton live male births. This may suggest a discrepancy in the supply of hCG to the fetus where a single placenta is unable to meet the requirements of two developing male fetuses.
Chordee
Ventral penile curvature (previously known as chordee) accompanies hypospadias in some cases. It is seen more commonly in severe cases of hypospadias but can also occur independent of hypospadias. Study of penile development via examination of fetal specimens has led to the understanding that chordee is a normal stage in penile development and that significant variation in the severity of chordee was noted at all stages of embryogenesis.
If curvature is an arrest of normal embryologic development analogous to failure of descent of the testicle, it is no surprise that fibrosis is conspicuously absent in some clinical cases of chordee. Snodgrass and colleagues further supported this by studying subepithelial biopsies of urethral plate examined under a microscope. They demonstrated well-vascularized connective tissue comprised of smooth muscle and collagen without evidence of fibrous bands or dysplastic tissue. Baskin and colleagues found well-vascularized connective tissue under the epithelial surface of the urethral plate in a 33-week fetus with distal hypospadias.
In some patients, curvature is present without hypospadias. Devine and Horton described three types of chordee without hypospadias. In class I, the most severe defect, the corpus spongiosum is deficient from the site at which the chordee begins, up to the glans, whereas the urethra has a thin tube of mucous membrane. In class II, the urethra has a normal corpus spongiosum with abnormal Buck’s fascia and dartos fascia layers. In class III, only the dartos fascia layer alone is abnormal.
Associated findings
Cryptorchidism and Inguinal Hernia
Between 8% and 10% of boys with hypospadias have a cryptorchid testicle and 9% to 15% have an associated inguinal hernia. In boys with more proximal hypospadias, cryptorchidism may occur as frequently as 32%. This strong association between proximal hypospadias and undescended testis further suggests that this clinical entity may represent one end of a spectrum of endocrinopathy. The incidence of chromosomal anomaly in these groups of patients is much higher (22%) than hypospadias (5%–7%) or cryptorchidism (3%–6%) occurring alone. In a series of more than 600 cases of hypospadias, the authors found that children with associated cryptorchidism and midshaft to distal hypospadias had a much higher complication rate when corrected. It is not certain why this occurs but it may be that a change in the endocrine milieu with the associated cryptorchidism may make the tissues less amenable to correction.
Disorders of Sex Development
Hypospadias and disorders of sex development (DSD) may represent two ends of a spectrum. The more severe the hypospadias, the more likely a DSD state exists. Rajfer and Walsh reported DSD in 27.3% of boys with a normal-sized phallus, cryptorchidism, and hypospadias. Presence of severe hypospadias and nonpalpable testes with otherwise normal-looking phallus requires that the urologist test for the presence of a DSD state.
Partial androgen insensitivity, chromosomal abnormalities, Smith-Lemli-Opitz syndrome, 5α-reductase deficiency, Denys-Drash syndrome, and other conditions can also occur in association with hypospadias.
Prostatic Utricle
The prostatic utricle is an elementary structure developing from the müllerian ducts cranially and from the wolffian ducts and the urogenital sinus caudally. Boys with hypospadias often have enlargement of the prostatic utricle with resultant urinary tract infections, stone formation, pseudoincontinence, and, often, difficult catheterization. Devine and colleagues reported that 57% of patients with perineal hypospadias and 10% with penoscrotal hypospadias had prostatic utricle enlargement demonstrated on urethroscopy. The overall incidence of utricle enlargement in patients with hypospadias was 14% in this series of 44 patients. Utricular enlargement in itself does not indicate a DSD but is seen with increased frequency in patients with 46 XY DSD.
Presentation
The abnormal dorsal prepuce and ventral glans tilt of the newborn penis usually signifies the presence of hypospadias. Further examination of the penis typically reveals the proximally displaced urethral orifice that is often stenotic in appearance but rarely obstructive. An exception is the megameatus variant of hypospadias. In this unusual case (6% of all distal hypospadias presentations), an intact prepuce is present. The diagnosis is usually not made until after a routine neonatal circumcision is completed.
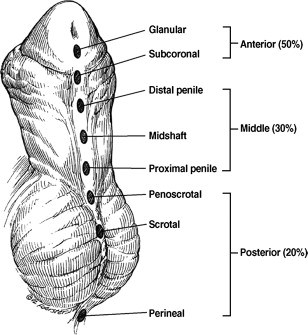
The anatomic location of the meatus and extent of ventral curvature, or chordee, should be determined. In some instances, multiple pinpoint dimples may be present on the surface of the urethral plate in addition to the hypospadiac urethral meatus. The meatus is always the most proximal of these defects. Meatal position may be classified as anterior (distal), middle, or posterior (proximal) with more anatomically specific subgroups further applied (see Fig. 2 ). The meatus is located on the glans or distal shaft of the penis in approximately 70% to 80% of all boys with hypospadias. Twenty percent to 30% of boys with hypospadias have the meatus located in the middle of the shaft of the penis. The remainder of boys with hypospadias has more severe defects with the urethral meatus located in the scrotum or even more proximally on the perineum.
Increased understanding of the endocrinologic origins of hypospadias has corroborated the clinical association of hypospadias with DSD states. Boys with severe proximal hypospadias and those with hypospadias and cryptorchidism should undergo karyotype analysis and a DSD evaluation as indicated. The unilaterality or bilaterality of cryptorchidism concomitant with hypospadias does not predict the diagnosis of a DSD state.
A complete penile examination requires independent evaluation of penile length. If the stretched penile length is significantly below the third percentile for age or if inadequate phallic size precludes surgical repair of hypospadias, then androgen stimulation as pretreatment should be considered. Androgenic pretreatment with hCG has been shown to increase penile length and may also move the meatus to a relatively more distal position as the shaft elongates in response to the hCG.
Surgical repair
The goal of hypospadias surgery is a functional sexual organ that is free of curvature. Equally important is a glanular urethral meatus that allows a boy to void with a laminar flow while standing. A cosmetically sound penis requires a cone-shaped glans and supple penile shaft skin.
Timing of Surgery
Historically, the American Academy of Pediatrics has stated that the ideal age for genital surgery is between 6 and 12 months of age. This age range seems to insulate most children from the psychological, physiologic, and anesthetic trauma associated with hypospadias surgery. The authors prefer, however, to perform the repair at the age of 4 months in infant boys with an adequately sized phallus and without medical problems. Surgery even earlier may be effective in boys with adequate glans volume. Healing seems to occur quickly, with less intense scarring, and young infants overcome the stress of surgery more easily.
Instruments
Increasing experience has demonstrated the applicability of the principles of plastic surgery to hypospadiology. Instruments, such as fine scissors, 0.5-mm tooth forceps, and Castroviejo needle holders, are standard. Fine absorbable 6-0, 7-0, or 8-0 sutures work well for suturing with precision. Considerable variation exists among surgeons as to the choice of suture material. The authors prefer polygycolic suture material for construction of the neourethra and for buried sutures and continue to use a glanular stay suture to minimize tissue handling during repair. The authors do not recommend polydiaxanone suture for urethral repair due to its extended absorption time interval and increased urethral stricture rate.
Hemostasis
Adequate hemostasis may be achieved by a variety of techniques in hypospadias surgery. A tourniquet placed at the base of the penis that is removed every 15 to 30 minutes alone or combined with needlepoint spot and bipolar electrocoagulation is often used to control blood loss that may occlude the surgical field. The authors avoid the use of electrocautery to minimize the potential for tissue damage caused by cautery dispersal and continue to inject 1:100,000 epinephrine in 1% lidocaine along the proposed incision line in the glans. In the authors’ experience, this injection affords the twin benefits of adequate local hemostasis while hydrodissecting a reliable dissection plane between the skin and dartos fascia.
Dressing and Urinary Diversion
An ideal posthypospadias repair dressing should provide adequate but pliable compression and be easily removable within 48 hours in most cases. Many variations in type and style of dressing have been proposed, although even no dressing at all is a viable alternative. The authors continue to prefer the sandwich-type dressing preferred by Duckett that compresses the penis against the lower abdominal wall by placing a Telfa (Kendall, Mansfield, MA, USA) pad and a folded gauze sponge on top of the penis followed by a bioocclusive dressing such as Tegaderm (3M, St Paul, MN, USA).
Urinary diversion is often preferred after proximal and midshaft hypospadias repairs, yet its usefulness in distal repairs is based on surgeon preference rather than proved benefit. A multicenter experience reported by Hakim and colleagues revealed similar results for distal repairs with or without postoperative urethral diversion. The authors use a 6 French (F) hydrophilic Kendall catheter placed through the neourethra and sutured to the glans with a prolene suture anchored to the inner aspect of the meatus to avoid scarring of the glans. Because the rate of urinary infection is no different between an open and closed system, the authors allow the open end of the Kendall tube to passively drain into the outer of two diapers and prescribe chemoprophylaxis.
Types of hypospadias repairs
Because hypospadias repair is so challenging, a plethora of surgical options, from those representing truly novel approaches to modifications of known procedures, have been described for various presentations of hypospadias. The surgical technique that is most appropriate for a given case is based on anatomic factors, previous surgical descriptions, and, of course, a surgeon’s personal experience.
Historically, hypospadias repairs were categorized as primary closures, meatal-based flaps, dorsally based flaps, and free grafts. Conceptual advances, such as recognition of the urethral plate and its potential for incision and preservation, have profoundly affected the approach to hypospadias repair today. This article describes the techniques implemented at the authors’ institution for hypospadias repair with an understanding that these descriptions are truly templates that are constantly modified, amplified, and reinvented—a practice that is the basis of evolution in hypospadiology.
Distal hypospadias
Meatal Advancement and Glanuloplasty
The meatal advancement and glanuloplasty (MAGPI) offers reliable cosmesis and long-term success when applied to repair glanular and selected coronal hypospadias. Presence of urethral mobility and a rounded glans along with the absence of significant chordee ensure an ideal outcome and avoid meatal regression.
The MAGPI begins with a circumferential incision 6 to 8 mm proximal to the corona of the glans and proximal to the meatus. Penile shaft skin is dissected in a drop-back fashion with extreme care exercised ventrally over the corpus spongiosum to avoid urethral injury. Residual chordee or penile torsion may be corrected at this point. A longitudinal incision from the dorsal distal edge of meatus is carried to the distal glans groove as it transects the transverse bridge of tissue that is often present. The incised tissue edges are approximated in a Heineke-Mikulicz fashion with 7-0 absorbable suture to effectively advance the meatus distally. The medial edge of the ventral meatus is then pulled distally and the exposed glans edges are trimmed and anastamosed to leave the glans with a cosmetically sound, rounded appearance. The dorsal hood foreskin is trimmed in the midline as Byars flaps allow adequate ventral skin transfer, and skin is approximated to the glans with absorbable, subcuticular suture to complete the repair.
The authors are using the MAGPI repair less and less. The tubularized incised plate urethroplasty (TIP) has become the standard for all but the most distal repairs.
Tubularized Incised Plate Urethroplasty
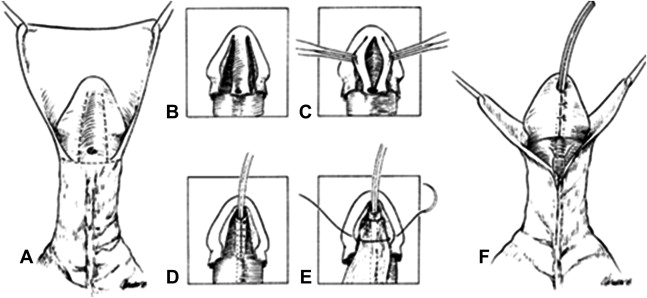
Recognition that surgical repair of a hypospadias based on a flat urethral plate resulted in a horizontal, recessed meatus in contrast to the cosmetically superior results when repairing a deeply grooved plate led Rich and colleagues to propose the “hinging of the urethral plate” by incising it distally. Snodgrass extended this concept by incising the plate deeply through the entire urethral plate to the corporal bodies followed by a Thiersch-Duplay tubularization. A multicenter experience supported the concept and was followed by the application of TIP to proximal hypospadias repairs.
A circumscribing skin incision is carried ventrally to 1 to 2 mm proximal to the urethral meatus and followed by skin drop-back to the penoscrotal junction ( Fig. 3 A). Penile curvature is resolved and corrected by dorsal midline plication, if necessary. Two parallel longitudinal incisions in the glans allow lateral mobilization of the glans wings as care is taken not to undermine the vascularity of the urethral plate (see Fig. 3 B).