Fig. 13.1
Age-specific prevalence of biochemically defined testosterone deficiency [Testosterone deficiency defined as total testosterone <300 ng/dL or current testosterone therapy. For every 10-year increase in age, risk of TD increased by 17 % (95 % confidence interval [CI], 1.08–1.27)]. Adapted from [6]
Table 13.1
Prevalence of concomitant cardiometabolic conditions in men with total testosterone less than 300 ng/dL
Risk factor | Hypogonadism prevalence (95 % CI) | Odds ratio (95 % CI) |
|---|---|---|
Obesity | 52.4 (47.9–56.9) | 2.38 (1.93–2.93) |
Type 2 diabetes | 50.0 (45.5–54.5) | 2.09 (1.70–2.58) |
Hypertension | 42.4 (39.6–45.2) | 1.84 (1.53–2.22) |
Hyperlipidemia | 40.4 (37.6–43.3) | 1.47 (1.23–1.76) |
Asthma or COPD | 43.5 (36.8–50.3) | 1.40 (1.04–1.86) |
Prostate disease | 41.3 (36.4–46.2) | 1.29 (1.03–1.62) |
The 2010 Endocrine Society Guidelines define hypogonadism as signs and symptoms of low testosterone (e.g., decreased libido, decreased erections, decreased energy, decreased physical strength) in the setting of low first-morning serum T levels defined as less than 300 ng/dL on two separate occasions [7]. TRT has been shown to reliably increase serum testosterone to physiologic levels, improve libido, improve erectile dysfunction, improve overall sexual function, increase energy, improve mood, increase bone mineral density, decrease body fat mass, and increase lean body muscle mass [7, 8]. The Boston Area Community Health (BACH) Survey estimated a crude prevalence of symptomatic androgen deficiency at 5.6 % [9], while it was estimated that only 12 % of symptomatic men were being treated [10]. These trials were the first to describe the magnitude of TD among the aging male population, relative to a decline in various levels of functioning.
Hypogonadism has been identified concomitantly with many comorbid health conditions in men including obesity, cardiovascular disease (CVD), MetS, type 2 diabetes mellitus (T2DM), hypertension (HTN), and dyslipidemia, positing an associative relationship. The exact physiologic mechanisms behind these proposed relationships have still not been inarguably determined. While it has been proposed that hypogonadism may be a direct cause of some or all of these comorbid conditions, a simultaneous condition associated with another underlying process such as senescence, or even a protective evolutionary factor that decreases energy expenditure in men with poor or declining health status, a definitive cause-and-effect answer still remains elusive [8, 11]. Despite such theories, men with hypogonadism have been shown to have less favorable health outcomes compared to the general population and thus may be more susceptible to potential adverse effects associated with TRT, through an associative relationship [7].
Pathophysiology
TD occurs due to a dysregulation in the hypothalamic–pituitary–gonadal axis and is a component of natural senescence in aging men. Decreased levels of gonadotropin-releasing hormone (GnRH) in the pituitary result in decreased levels of serum luteinizing hormone (LH) secreted by the pituitary that in turn directly impact testosterone production at the level of the testes. This is associated with increased serum insulin (insulin resistance) and decreased sex hormone-binding globulin (SHBG). TD results in increased lipoprotein lipase, increased uptake of serum triglycerides, and further increases in visceral adiposity. The final result from this metabolic derangement is an increase in serum aromatase and estrogens that are deposited in visceral fat [14].
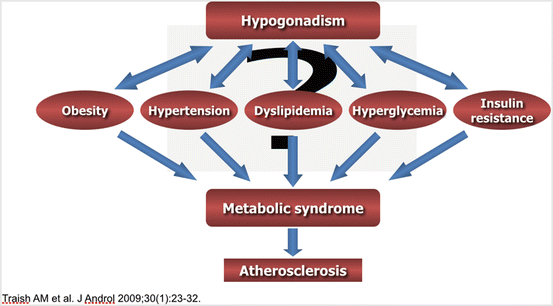
Relationship to Metabolic Syndrome
MetS poses a substantial threat to the health of millions of men worldwide, as it has been implicated in adverse morbidity and mortality cardiovascular outcomes. Although no single universally accepted definition exists, the syndrome is ubiquitously characterized by major cardiovascular risk factors including hypertension, hyperlipidemia [specifically elevated triglycerides and reduced high-density lipoprotein cholesterol (HDL-C)], increased fasting glucose, hyperinsulinemia, and increased visceral adiposity [12] (Fig. 13.2). These factors ultimately increase the risk of CVD and T2DM. This syndrome is derived from physical inactivity; obesity; a high-carbohydrate, high-fat diet; and genetic factors. Research over the last decade has examined the role of MetS and testosterone deficiency. MetS has been found to be associated with hypogonadism and erectile dysfunction (ED); thus, MetS is likely a risk factor for the development of ED and underlying endothelial dysfunction [13].
A study by Zitzmann highlights the relationship between hyperinsulinemia and visceral obesity leading to a reduction of endogenous testicular testosterone production [14] (Fig. 13.3). Testosterone has been shown to have negative reciprocal effects on generation of muscle and visceral adipose tissue through influencing the differentiation of pluripotent stem cells and through inhibiting the development of preadipocytes. Testosterone has a protective effect on pancreatic beta cells, exerted by the positive influence of inflammatory cytokines and androgen receptor-mediated pathways. Several epidemiological and interventional studies have posited that TRT may be integral in either preventing or attenuating the MetS in aging men with LOH and in those with concomitant hypogonadism and T2DM [14].
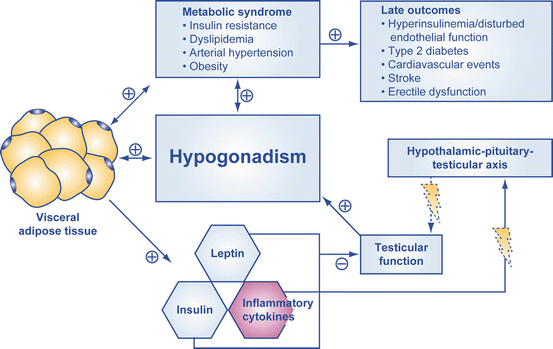

Fig. 13.3
Visceral adiposity is a key component of metabolic syndrome. Lightning signs indicate a disturbance of function. Adapted from [14]
Traish and colleagues have written a series of publications highlighting “the dark side” of the relationship between testosterone deficiency and the metabolic syndrome. This relationship has defined a proven link among reduced plasma testosterone levels, T2DM, and insulin resistance. TRT in the treatment of hypogonadal men has been shown to improve insulin sensitivity, serum fasting glucose levels, and glycosylated hemoglobin levels. Testosterone deficiency has been found to be associated with all of these components, as well as with an increased deposition of visceral fat, which serves as an endocrine organ producing inflammatory cytokines and thus promoting endothelial dysfunction and vascular disease [13, 15].
Measurement of Serum Testosterone
Various assays exist to measure serum testosterone (Fig. 13.4) [16]. Most current literature focuses on measurement of total testosterone, yet bioavailable testosterone assays are becoming more popular as a modality toward more specific identification of the hormone. Most circulating testosterone is bound to sex hormone-binding globulin (SHBG), while approximately 0.5–2 % is unbound to either SHBG or albumin and is considered as free testosterone. All testosterone that is not bound to SHBG is considered to be bioavailable, estimated at approximately 40–50 % of total.
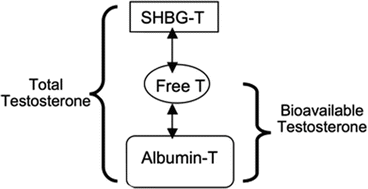

Fig. 13.4
Composition of serum testosterone. SHBG sex hormone-binding globulin, T testosterone. Adapted from [16]
Evaluation and Treatment of the Man with Suspected Testosterone Deficiency
Significant controversy exists over whether or not a man should be screened for potential TD in primary care practices. A recent pro/con debate by Heidelbaugh (pro) and Fugh-Berman (con) published in the American Family Physician journal in 2015 highlights the key tenets of this argument (Table 13.2) [17, 18]. A challenge with identifying appropriate symptoms of the man who presents with potential TD lies within the often nonspecific symptoms associated with this disorder (Table 13.3) [7, 19, 20] (Table 13.3) and associated with the comorbid disease states establishing high risk. The Endocrine Society advises against generalized screening. The authors believe that all men who present with largely “organic” ED should be screened with a total testosterone. The Androgen Deficiency in the Aging Male (ADAM) Questionnaire is a validated tool that can help clinicians to screen men appropriately for TD (Table 13.4) [21].
Table 13.2
Should clinicians screen for testosterone deficiency in men?
Yes |
Testosterone levels decline as men age |
Testosterone deficiency is a real syndrome with real symptoms and improvable metabolic outcomes |
Studies suggesting cardiovascular risk associated with TRT have major flaws |
TRT has proven benefit in cardiometabolic syndrome |
No |
Aging adults are a profitable market; TRT has been promoted as a “youth-restoring tonic and disease preventive” |
“Pharmaceutical companies use nonspecific symptoms to foster disease states” |
No consistent relationship has been proven between testosterone levels and symptoms associated with low testosterone |
Table 13.3
Symptoms and signs suggestive of testosterone deficiency in men
More specific signs and symptoms |
Reduced libido |
Erectile dysfunction (ED) |
Reduced intensity of orgasm and genital sensation |
Osteoporosis or low bone mineral density |
Decreased spontaneous erection |
Oligospermia or azoospermia |
Very small or shrinking testes |
Hot flushes, sweats |
Breast discomfort, gynecomastia |
Loss of pubic and axillary hair, reduced shaving |
Less specific signs and symptoms |
Decreased energy or vitality, increased fatigue |
Depressed mood |
Reduced muscle mass and strength |
Poor concentration and memory |
Sleep disturbance; increased sleepiness |
Mild anemia |
Increased body fat, body mass index |
Diminished physical or work performance |
Table 13.4
ADAMa Questionnaire
1. Do you have a decrease in libido? |
2. Do you have a lack of energy? |
3. Do you have a decrease in strength and/or endurance? |
4. Have you lost height? |
5. Have you decreased an “enjoyment of life?” |
6. Are you sad and/or grumpy? |
7. Are your erections less strong? |
8. Have you noted a decrease in ability to play sports? |
9. Are you falling asleep after dinner? |
10. Has there been a recent deterioration in work performance? |
A significant number of comorbid conditions coexist with TD (Table 13.5) [7]. In men with these conditions, a workup for TD should be strongly considered, especially when concomitant conditions have become difficult to manage (e.g., T2DM, morbid obesity, and other endocrine conditions). Testosterone deficiency is classified as primary, secondary, and mixed disorders (Fig. 13.5) [7]. It is imperative that clinicians appropriately characterize and classify TD in men to propose appropriate treatment.
Table 13.5
Risks and comorbid illnesses associated with testosterone deficiency
Metabolic syndrome |
Obesity |
Hyperlipidemia |
Hypertension |
Elevated fasting plasma glucose and serum insulin |
Diabetes mellitus (type 1 or 2) |
Cardiovascular disease (including aortic atherosclerosis) |
Elevated C-reactive protein |
Chronic obstructive lung disease |
Inflammatory arthritis |
Low-trauma fracture |
End-stage renal disease |
HIV-related weight loss |
Hemochromatosis |
Sellar mass, radiation to the sellar region, or other diseases of the sellar region |
Chronic pain syndrome and treatment with opioids |
Treatment with glucocorticoids |
Radical prostatectomy |
The Endocrine Society Guidelines from 2010 present a schematic to aid clinicians in the evaluation of the man with suspected TD (Fig. 13.6) [7]. By convention, a cutoff of 300 ng/dL has been defined as TD, coupled with the symptoms highlighted in Table 13.3. Currently, there are a variety of widely available testosterone formulations including topical gels and patches, intramuscular injections, subcutaneous pellets, and oral/buccal formulations that provide clinicians and male patients the opportunity to personalize replacement therapy.
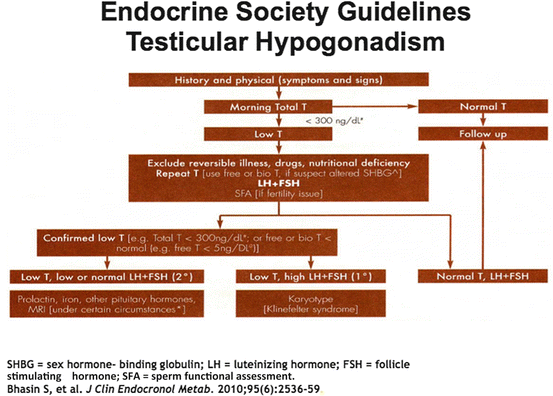

Fig. 13.6
Endocrine Society Guidelines for testicular hypogonadism. SHBG sex hormone-binding globulin, LH luteinizing hormone, FSH follicle-stimulating hormone, SFA sperm functional assessment. Adapted from [7]
Potential Adverse Effects of Testosterone Replacement Therapy
The estimated likelihood of adverse effects of long-term TRT is still essentially unknown, as overall high-quality evidence to recommend its use in most men with TD is lacking. The highlighted studies can be used to guide the clinician in how to best monitor patients on TRT, especially those with the comorbid conditions detailed below.
Risk of Cardiovascular Morbidity and Mortality
Reduced serum testosterone levels have been associated with an increased risk of the development of CVD including ischemic heart disease and stroke, yet whether TD is directly linked to the pathogenesis of CVD, a marker of preexisting CVD, or concomitant manifestation of another underlying disease remains unclear [8, 11]. Low endogenous testosterone levels correlate with an increased risk of adverse CVD events, and endothelial dysfunction and increased atherosclerosis are means by which male hypogonadism may contribute to an increased risk of death [22]. The potential that TD may be involved in the pathogenesis of CVD would create a notion that TRT would result in improved cardiovascular outcomes, yet no current level I evidence exists to support this claim. There are several well-done cross-sectional studies that suggest this association between TRT and improved mortality including CVS mortality.
To date, the literature on the relationship between TRT and CVD has been conflicting, suggesting that TRT has either no beneficial effect on reduction of cardiovascular morbidity or mortality or even a detrimental effect. Two meta-analyses found no differences in cardiovascular events between TRT and placebo groups [23, 24], while a more recent meta-analysis found that TRT increased the risk of cardiovascular events, although the data varied by source of research trial funding. The authors concluded that overall, and particularly in trials not funded by the pharmaceutical industry, exogenous testosterone increased the risk of cardiovascular-related events [25].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree