Fig. 18.1
Pathway of malnutrition and gastrointestinal disorders in children with HIV infection. A complex interplay exists among these conditions. (Reprinted from [3] with permission)
Since the beginning of AIDS pandemic, it was clear that the virus gains access to the human host predominantly through the mucosal tissue after sexual exposure. However, HIV mucosal infection plays a critical role not only in virus transmission but also in AIDS pathogenesis, affecting mucosal surface of the gastrointestinal (GI) tract in the early phase post infection and depleting cluster of differentiation (CD4) T helper cells [4]. GI disease accounts for a high proportion of presenting symptoms of HIV infection, mainly in developing world [5]. Chronic diarrhea is a hallmark of advanced HIV infection, and it is caused by intestinal infections. Although the widespread use of HAART has dramatically improved the survival rate of HIV-infected people and has changed the spectrum of complications of HIV disease, the intestinal tract is still a major target of HIV infection. An uncertain, but high, percentage of HIV-infected patients worldwide initially presents or ultimately develops diarrhea or malnutrition , with or without HAART. Diarrhea is a major manifestation of HIV disease and is associated with increased morbidity and mortality. It substantially increases health-care costs for many patients, particularly those with severe malabsorption and malnutrition requiring repeated hospitalizations. In many cases, a multidisciplinary approach including specialist in gastroenterology, infectious disease, especially HIV, oncology, surgery and microbiology is required for treating patients with AIDS.
The Spectrum of GI Disorders in HIV Infection
The stage of immunodeficiency, reflected by the CD4 lymphocyte count, should be kept in mind when approaching any HIV-infected patient with GI symptoms because the etiology and the frequency of opportunistic infections are related to the CD4 count and rises exponentially if CD4 is < 100 cell/ul.
The upper GI tract is a topographical target of HIV infection. Candida is the most common oesophageal pathogen in AIDS. However, in the era of antiretroviral therapy (ART), many of the previously common HIV-related disorders are vanished [6], but they can be observed in patients who are failing on HAART or non-adherent to therapy [7]. The approach to HIV patients with a CD4 count > 200 cell/μl and upper GI symptoms should parallel that of any other non-HIV-infected subject. Upper endoscopy remains the gold standard diagnostic approach in case of suspected gastro-oesophageal reflux disease, dysphagia or bleeding. In severely immunosuppressed children, in whom opportunistic infections are considered, upper endoscopy with multiple tissue sampling should be performed for a definitive diagnosis [8]. For example, multiple biopsies (about 10) increase the yield for cytomegalovirus (CMV) disease [9].
Intestinal infections are a major cause of morbidity and mortality, especially for children, and where the availability of HAART is limited. In patients with advanced HIV disease, opportunistic infections are the most common cause of disease. Cryptosporidium and microsporidium are the most common pathogens in developing countries and CMV in developed world. However, although opportunistic causes of diarrhea have fallen dramatically in the era of ART, overall the number of patients experiencing diarrhea has changed very little [10]. Other infections unrelated to immunodeficiency and noninfectious causes are also increased in HIV-infected children. Drug-induced diarrhea is an important cause of diarrhea in HIV patients [11]. Severe diarrhea associated with villous atrophy and crypt hypertrophy with no pathogen is found despite extensive investigation is defined as HIV enteropathy. The approach to diarrhea in HIV-infected patients should be based on routine stool testing, but these are often repeatedly negative, mainly in severe immunosuppressed subjects. In these cases, endoscopic examination with biopsy is able to establish a diagnosis in 65–80 % of cases [8].
Intestinal dysfunction is a specific HIV-related syndrome in children [3]. The clinical manifestations of intestinal dysfunction may be limited or absent. Namely dysfunction is not consistently associated with diarrhea but its most prominent features are steatorrhoea, reduction of the intestinal absorptive surface and increased permeability [3]. Despite the limited or absent clinical manifestations of intestinal dysfunction, nutrient malabsorption certainly contributes to weight loss, and its clinical consequences may be expected in the long term and associated with progressive failure to thrive. The pathophysiology of intestinal dysfunction is complex and involves multiple abnormalities. HAART is able to restore intestinal function tests in HIV-infected children, in parallel with a decrease in viral load and an increase in CD4 T cell count [3]. This suggests that in case of malnutrition in a child receiving ART, careful monitoring of antiretroviral therapeutic efficacy is needed, as malnutrition may be an early marker of treatment failure.
Liver disease is the second most common cause of death among adults with HIV infection. Just as the burden of non-AIDS morbidity and mortality has changed in the ART era, the spectrum of liver diseases has also changed in these patients [12]. Prior to ART, the most common causes of liver dysfunction were opportunistic infections , including CMV and mycobacterium infections, AIDS lymphoma and Kaposi’s sarcoma. Since the HAART era, however, the spectrum of liver disease among HIV-infected individuals has shifted to concomitant infection with chronic hepatitis C virus (HCV), and chronic hepatitis B virus infections, medication-related hepatotoxicity and nonalcoholic fatty liver disease (NAFLD) . Although hepatic dysfunction is not frequent in HIV in children, hepatomegaly without other symptoms of systemic disease is commonly found in pediatric patients, and it is often associated with nutritional deficiency. HBV and HCV infections may be more severe in HIV-infected children than in HIV-negative individuals. In addition, liver toxicity is one of the most common serious adverse events associated with ART [12].
Pancreatic dysfunction may be considered as a component of HIV-associated digestive dysfunction in HIV. In fact, a reduction of faecal levels of pancreatic elastase and/or chymotrypsin has been found in one third of HIV-infected children [13]. The clinical manifestations of pancreatic involvement may not be evident, because exocrine, rather than endocrine pancreatic function, is involved. Pancreatitis may be an unusual but serious drug adverse effect, mainly with selected nucleoside analogue reverse transcriptase inhibitors.
HIV Enteropathy
Since the discovery of HIV as an agent of AIDS , histological abnormalities of GI tract were observed, and the term “HIV enteropathy” was firstly used in 1984 [14] . Many investigators found that HIV itself infected enterocytes, lamina propria and submucosal cells. The enteropathy was characterized by inflammatory infiltrates of lymphocytes, damage to the GI epithelium, villous atrophy, crypt hyperplasia, and villous blunting, in the absence of detectable enteropathogens, recurring in all stages of HIV disease [15]. GI abnormalities associated with HIV infection include a decreased capacity for epithelial regeneration, impaired absorptive ability of GI mucosa, increased mucosal permeability, dysregulations of genes associated with T cell homeostasis, decreased growth factor production and cell-cycle mediators and upregulation of genes associated with apoptosis [16–18] .
Not surprisingly, inflammatory changes are mild or absent. Once opportunistic disorders are excluded in patients with refractory diarrhea, HIV enteropathy is diagnosed in a small but definite percentage of patients. The HIV enteropathy encompasses an idiopathic, pathogen-negative diarrhea, and it can occur from the acute phase of the HIV infection through advanced disease, leading to GI inflammation, increased intestinal permeability, bile acid and vitamins malabsorption [19]. The functional disruption of the GI caused by HIV has been reported also in the absence of clear clinical GI manifestations in the era of modern ART [20]. More than one third of HIV-infected patients present an abnormal D-xylose test, about 20 % has low or borderline serum B12 levels and 7 % has low albumin levels, in the absence of stool pathogens [20].
Although the mechanisms responsible for these abnormalities remain not completely known, several explanations have been put forward, from a virotoxic effect of HIV itself on enterocytes [21, 22] to an abnormal differentiation of enterocytes induced by HIV [23, 24], to local activation of the GI immune system [25]. However, a major role is likely associated with HIV transactivator factor (Tat) [21] .
Interaction Between HIV and Intestine
Immunopathogenesis of HIV involves two phenomenons: the loss of lymphoid cells and the loss of immune function. Several pathological changes, both structural and immunological, occur at the intestinal mucosal surface from the initial stage of HIV infection. The intestinal mucosa is a target for HIV and a site of significant HIV replication and CD4 T cell destruction, based upon the route of exposure and other aspects of mucosal immunology [26, 27]. Gut-associated lymphoid tissue (GALT) was identified as an early site of HIV replication and CD4 T cells depletion since the initial stage of HIV infection [28]. Even with early and aggressive HAART, GALT is not preserved and undergoes significant damage.
The sensitivity by intestinal tract to HIV infection relates in part to the high number of activated memory T cells expressing C-C chemokine receptor type 5 (CCR5) chemokine receptors located within the gut. HIV-1 isolated from acutely infected subjects are predominantly R5 viruses, that is macrophage-tropic HIV that requires CCR5 for cell entry, in contrast to R4 virus, lymphocyte-tropic HIV requiring the C-X-C chemokine receptor type 4 (CXCR4) chemokine receptor [29]. The preferential loss of CCR5 + CD4 T cells from the GI tract clearly suggests a virus-centric mechanism. Furthermore, the intestinal mucosa has continuous exposure to antigens, leading to a pro-inflammatory state, and secretes many cytokines that stimulate HIV replication [18].
There is a compartimentalization of HIV infection between blood and mucosa, mainly due to viral factors. The CD4/CD8 ratio was analysed in the duodenum and peripheral blood in order to compare CD4 T cell depletion between these two anatomical sites, and it was found that the GI tract was preferentially depleted of CD4 T cells [30–33]. Moreover, CD4 T cells in the GI tract are tenfold more frequently infected by HIV than those in the peripheral blood, suggesting that the viral reservoir in the intestine is much greater than previously thought [34].
Another mechanism that can contribute to the HIV enteropathy is the infection of enterocytes by HIV 1. In in vitro experiments, HIV is able to replicate in the intestinal epithelial cell lines [35, 36]. However, virus-direct and -indirect effects have been described. In addition to structural and enzymatic proteins, HIV-1 encodes a group of at least six auxiliary regulatory proteins, including Tat , a transactivating peptide essential for HIV replication, which exerts its effects by activating L-type Ca2+ channels, and/or mobilizing intracellular calcium stores [37, 38]. Tat is secreted from HIV-1-infected cells and is taken up by neighbouring uninfected cells. In vitro experiments using human colonic adenocarcinoma (caco-2) cell monolayers and human colonic mucosa specimens mounted in ussing chambers, it has been shown that Tat protein induced ion secretion similar to that induced by bacterial endotoxins. It also significantly prevents enterocytes proliferation [39]. On the bases of these data, it is likely that Tat directly exerts pathogenic effects on enterocytes and is involved in the pathogenesis of intestinal mucosal atrophy typical of HIV-infected patients, that is, in HIV enteropathy .
Tat has been implicated also in enterocyte apoptosis. Tat causes an imbalance in reactive oxygen species (ROS) generation in neurons, which is neutralized by antioxidants, thereby implicating perturbation of the intracellular redox status in the pathogenesis of HIV-induced cell damage [40]. Oxidative stress is implicated in the pathogenesis and morbidity of HIV infection. An increase of ROS and an alteration of antioxidant defences have been reported in HIV-infected patients [41] associated with decreased levels of antioxidants [42]. The mechanisms involved in HIV-induced oxidative stress are unknown, but HIV-1 proteins gp120 and Tat have been implicated in this process because they both induce oxidative stress and cause apoptosis in brain endothelial cells [43]. Similar mechanism has been involved in intestinal mucosa (Fig. 18.2). In an in vitro cell model, Tat increased the generation of ROS and decreased antioxidant defences as judged by a reduction in catalase activity and a reduced glutathione (GSH)/oxidized glutathione disulfide (GSSG) ratio [44]. Tat also induces cytochrome c release from mitochondria to cytosol, and caspase-3 activation. Rectal dialysis samples from HIV-infected patients were positive for the oxidative stress marker 8-hydroxy-29-deoxyguanosine. GSH/GSSG imbalance and apoptosis were observed in jejunal specimens from HIV-positive patients at baseline and from HIV-negative specimens exposed to Tat. Experiments with neutralizing anti-Tat antibodies showed that these effects were direct and specific. Pretreatment with N-acetyl cysteine (NAC) prevented Tat-induced apoptosis and restored the glutathione balance in both the in vitro and the ex vivo model [44]. These findings indicate that oxidative stress is one of the mechanism involved in HIV intestinal disease (Fig. 18.3).
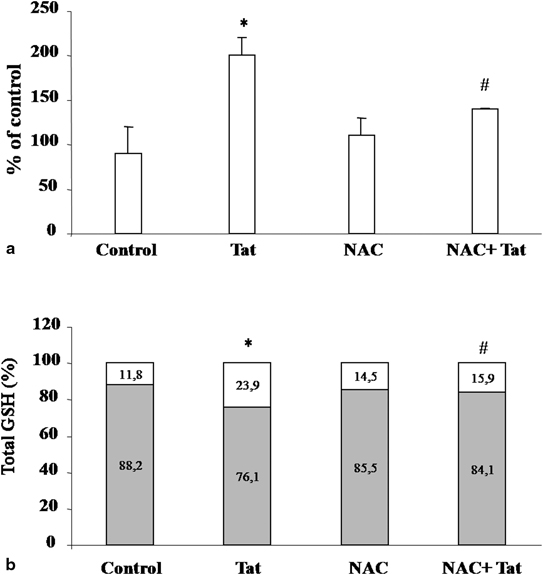
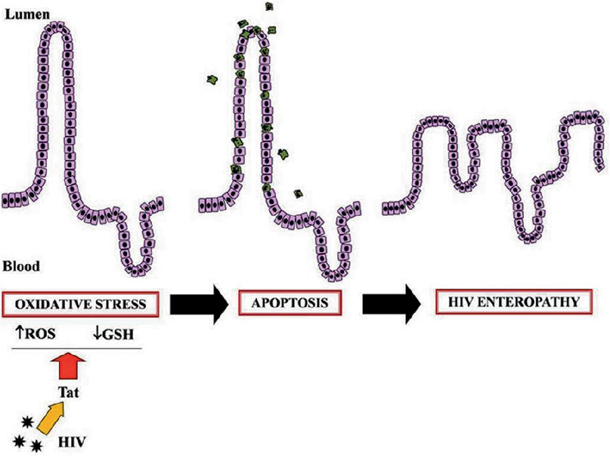

Fig. 18.2
Effect of NAC on the Tat-induced oxidative stress in Caco-2 cells [44]. Intracellular ROS levels, determined by fluorometric method, after exposure of Tat with or without pretreatment with NAC (a). Data were represented as percent of controls. Effect of NAC on Tat-induced GSH/GSSG imbalance (b). Data are represented as percent of GSH (grey) and GSSG (white) versus total glutathione. *p, 0.05 vs. control; #p, 0.05 versus Tat. Data are representative of three separate experiments. NACN-acetyl cysteine, Tat transactivator factor. (Reprinted from Ref. [44])

Fig. 18.3
Schematic representation of the mechanism of HIV Tat viral protein-induced oxidative damage to the intestinal mucosa [44]. Tat induces oxidative stress by increasing the ROS intracellular level and deranging the GSH/GSSG ratio. This leads to programmed cell death (apoptosis) and an increase in epithelial damage. Together with ion secretion and altered glucose transport, these steps could represent key mechanisms in HIV enteropathy. ROS reactive oxygen species, GSH glutathione, Tat transactivator factor, HIV human immunodeficiency virus . (Reprinted from Ref. [44])
HAART and HIV Enteropathy
HAART reduces viral loads and increases CD4 T cells. GI symptoms including abdominal pain , bloating and diarrhea improve after initiation of treatment, in parallel with CD4 T cell reconstitution in blood and a decrease in viral load in rectal tissue [36]. The effects of HAART on intestinal CD4 T cells are lower than in peripheral blood [45, 46]. Furthermore, HAART started early during infection leads to an increase in CD4 central memory cells in Peyer’s patches , but does not restore effector memory cells in the ileal lamina propria [47, 48]. Initiation of HAART during chronic infection does not significantly increase CD4 T cells in gut-inductive or -effector sites [45, 46]. Finally, no HIV-infected individual reconstituted GI tract CD4 T cells to levels observed in uninfected persons. These findings suggest that fibrotic damage severely disrupt the ability of GALT to support normal T cell trafficking and survival, even after HAART onset. In addition, not only CD4 T cells fail to fully repopulate the GI tract following HAART but also HIV proviral DNA persists in CD4 T cells in terminal ileum 10 years after effective therapy [49]. As a consequence, viral replication persists at low levels in the intestine despite HAART .
GI Infections
The spectrum of HIV-related GI infections has changed dramatically after the introduction and the worldwide diffusion of ART, and still today the epidemiology significantly varies between HIV-infected children treatment-naïve or on ART. The CD4 lymphocyte count continues to be the best predictor of the risk of an opportunistic infection [8, 50], although HIV viral load seems to be also helpful in predicting this risk [51, 52] (Fig. 18.4). Data in children on ART living in developed areas are limited to small reports; however, studies conducted in Africa and reporting the pathogens isolated in children presenting with HIV and diarrhea are reasonably consistent.
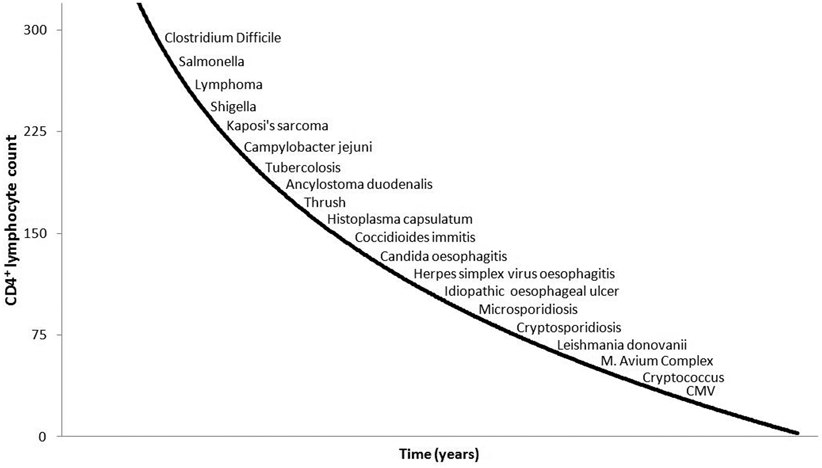

Fig. 18.4
Correlation between CD4 count and risk of HIV-related diseases. CD cluster of differentiation, CMV cytomegalovirus
A list of the most common agents in HIV/AIDS children is reported in Table 18.1. In addition, Kaposi sarcoma and intestinal lymphoma are also specifically included in HIV-associated intestinal disorders.
Table 18.1
Opportunistic disorders affecting GI tract in HIV/AIDS patients
Viral Agent | Cytomegalovirus, herpes simplex, rotavirus and adenovirus |
Parasites | Cryptosporidia, giardia and isospora |
Fungi | Candida, cryptococcus, microsporidia and histoplasma |
Bacteria | Shigella, listeria, salmonella, mycobacteria |
Tumour | Kaposi, lymphoma |
In addition, HIV may probably act as a direct enteropathogen and induces ulcerative processes involving distal oesophagus, ileum and rectum because only viral particles have been found in these lesions [53]. Different immunosuppressive approach including with thalidomide, anti-tumour necrosis factor (TNF) agents or corticosteroids have been used, in some case with success, to treat these lesions [54].
Upper Gastrointestinal Infections
The upper GI tract, including oral cavity, oesophagus and stomach, is commonly involved in HIV-related diseases.
Oesophageal localization of opportunistic agents is common in advanced HIV infections, and candida and herpes are most common agents of oesophageal ulcerations. This complication is strongly related to the immunological states, being quite common in subjects with CD4 count < 200 cells/μl, who are failing ART or are non compliant.
Oral thrush and odynophagia or dysphagia are the most commonly reported upper GI symptoms in HIV-infected children and adolescents. Thrush, appearing white–yellow, hard-to-remove plaques due to Candida infection, is a characteristic sign in young HIV/AIDS children. Candidiasis of the mouth and distal oesophagus are associated with loss of appetite, dysphagia and weight loss. Most severe cases of Candida infection cause necrotizing oesophagitis, bleeding and, occasionally, perforation. Other opportunistic agents may present with a similar clinical and endoscopic feature, thus biopsy is usually needed to exclude agents such as herpes, Cytomegalovirus (CMV) or intracellular Mycobacterium avium. Before ART introduction, Candida was by far the first cause of oesophageal complaints and empirical treatment with fluconazole, instead of endoscopy, was considered as the best strategy, especially in patients with thrush [55]. Recently, a report of more than 400 GI endoscopies in HIV/AIDS patients found that odynophagia/dysphagia had the highest yield of positive diagnosis, and it showed that both Candida and CMV are major agents of oesophageal involvement [54]. A large part of endoscopies, however, resulted in nonspecific oesophageal ulcers, pathophysiology of which is still unclear, and probably due to herpes or HIV itself.
Symptoms of dyspepsia , including epigastric pain, nausea and vomiting, are frequently reported by HIV-infected patients and especially those undergoing ART. The diagnostic approach in these patients may be challenging. Noninvasive testing for Helicobacter pylori is not useful in the diagnosis of HIV-infected subjects with strongly suppressed immune system , and a low incidence of this infection has been reported in this population [56, 57]. Empirical treatment with proton-pump inhibitors may be inappropriate for HIV-infected patients because these drugs reduce the oral absorption of selected protease inhibitors [58]. Atypical presentation of opportunistic infections should always be considered in this population and upper endoscopy is suggested for differential diagnosis. Multiple biopsies are needed since macroscopic normal-appearing mucosa does not exclude opportunistic infections [59]. However, the role of opportunistic infections in HIV/AIDS patients presenting with dyspepsia as primary symptom is still not clear. The rate of opportunistic infections in a large population of adults on ART presenting with dyspepsia was less than 2 %, and CMV was the most prevalent opportunistic agent [59]. Chronic nonspecific gastritis (mononuclear cell infiltrate) is the most common diagnosis in patients presenting with this symptomatology.
Diarrhea
Chronic diarrhea , in most of cases due to GI infections, is a hallmark of advanced HIV infection and remains a major substantial cause of morbidity and mortality in children.
In Africa, chronic diarrhea has been reported in as many as 90 % HIV-positive children, being five- to sixfold higher than in non-HIV-infected children [60, 61]. Case series from industrialized countries in the pre-ART era showed that 40–80 % of HIV-infected patients experienced diarrhea [62].
Classical and opportunistic enteropathogens may induce diarrhea in HIV-infected children. The duration and severity of diarrhea are increased in HIV-infected children living either in developing or in industrialized countries.
The likelihood of opportunistic infections is linked to the severity of immunodeficiency. Some studies [63] suggested that in immunocompetent individuals (CD4 > 200 cell/μl), the infections are usually caused by ordinary pathogens, while in those with a CD4 < 200 cell/μl, opportunistic agents such as Cryptosporidium parvum, CMV and Mycobacterium avium are more common. Frequent agents of diarrhea in HIV-infected subjects, such as the parasite Enterocytozoon bieneusi or Microsporidium, are more restricted to adult population.
Bacterial Diarrhea
Although there is no specific hallmark of bacterial etiology , several bacterial agents may induce diarrhea. The overall incidence of bacterial diarrhea in HIV-infected adults is at least 100-fold greater than in general population [64], and infections in HIV-infected subjects tend to be more protracted and severe than in immunocompetent individuals. The rate of diarrhea due to bacterial causes significantly decreased after the introduction of ART.
HIV-positive patients are at higher risk of prolonged and severe infections from Campylobacter jejuni and invasive non-typhoid Salmonellae infections. The risk of multisite Salmonella infections may be 300-fold increased in advanced HIV disease, and this condition has been considered an AIDS-defining illness .
Escherichia coli, Shigella and Clostridium were recognized to cause more frequently diarrhea in HIV-infected patients. Recently, a large retrospective study in the USA reported Clostridium difficile as the most common cause of diarrhea in HIV-infected adults, accounting for more than 50 % of cases [64]. In the same population Shigella, Campylobacter and Salmonella were found in 14, 13.8 and 7.4 % patients with diarrhea, respectively .
Mycobacteria may cause GI infections presenting with diarrhea. Diarrhea is a relatively uncommon symptom of Mycobacterium tuberculosis infection, but may be more common in Mycobacterium avium complex (MAC) infection. However, prophylaxis with azithromycin routinely performed in the pre-ART era is no longer indicated in children receiving ART.
Bacterial diarrhea may have a severe course in HIV-infected children, and it should be treated aggressively. The use of specific antibiotics should be carefully considered even in children who show a mild course of the disease, particularly in those who have moderate-to-severe immune impairment. Some children may require empiric treatment for symptom control or prevention of the risk of extra-intestinal spreading of the infection .
Viral Diarrhea
Viruses are the leading cause of acute diarrhea in children, and they usually have an acute and self-limiting course in immunocompetent children. Etiology of acute diarrhea in HIV-infected and non-infected children is substantially similar after the introduction of ART. A very high number of enteric viruses were detected in the stools of HIV-infected children without clear relationship with intestinal symptoms. However, viral enteropathy may be dangerous in HIV-positive children.
Rotavirus is the major cause of viral diarrhea in countries in which specific vaccination has not been routinely introduced. Children with HIV infection experience more frequent and severe Rotavirus infections, with a higher risk of prolonged hospitalization and a higher fatality rate than non-HIV-infected children [61] .
The development, validation and large-scale implementation of anti-Rotavirus immunization is changing the epidemiology of intestinal infections in industrialized countries that developed a routine vaccination programme [65]. Immunogenicity of Rotavirus vaccines in areas with high rate of HIV infection, like Africa, showed a good and consistent immune response. Immunogenicity is variable and resulted comparable or lower than those observed in Europe and the USA according to different evidence [66]. Although HIV infection is generally not a contraindication for immunization, high HIV prevalence in the region may result in lower rates of vaccine immunogenicity, efficacy and population immunity [67].
Other enteropathogens may cause acute diarrhea in HIV-infected children. Norovirus , generally considered the second leading agent of acute diarrhea, is fast becoming a major cause of medically attended gastroenteritis in countries with high Rotavirus vaccine coverage [68]. This agent may also induce severe and chronic disease manifestations in HIV-positive children [69].
CMV may act as an opportunistic enteric agent inducing severe colitis or enterocolitis or even an intractable diarrhea syndrome in severely immunocompromised children. CMV is by far the major opportunistic etiology of colitis in HIV-infected subjects [54]. As for other opportunistic infections, the introduction of ART significantly reduced the rate of CMV infection in adult patients decreasing from 7.34 to 0.75/100 patients-year. Also the survival at 1 and 2 years that was 42.6 and 16.7 % before ART, significantly improved to 84.6 and 76.9 %, respectively, in ART era [70].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







