Fig. 1.1
Design drawings of the lost da Vinci writings (from Institute and Museum of the History of Science, Florence, Italy). With permission from Ilya A. Volfson, Jeffrey A. Stock . History of Robotic Surgery. In: Jeffrey A. Stock MD, Michael P. Esposito MD, Vincent J. Lanteri MD, David M. Albala MD, edds. Current Clinical Urology: Urologic Robotic Surgery. Springer, New York 2008: pp 3–25. © Springer
The modern concept of the surgical robot had its origins in the development of virtual reality based on NASA’s use of a head-mounted display for visualizing the data being returned from Voyager’s exploratory mission. Scott Fisher and Joe Rosen combined this with the use of robotic waldoes and coined the term “telepresence,” founding Telepresence Research Inc., envisioning the use in telepresence surgery. Rosen and Fisher collaborated with Phil Green, a PhD at Stanford Research Institute, and, with other roboticists, worked to develop an interface allowing surgeons to operate virtually, with the sense that their robotic hands are directly in front of their eyes [2].
During this time, in the late 1980s, laparoscopy was also becoming mainstream, and the concept of a potential remote interface became a reality. It seemed that robotics could provide assistance to make up for the loss of three-dimensional visualization and impairment of dexterity that laparoscopy caused.
In 1985, Unimation’s PUMA 560 system, the first non-laparoscopic robotic device, was used to perform the first documented robotic-assisted surgical procedure, when it was used for a percutaneous brain biopsy (Fig. 1.2) [3]. In the early 1990s, Hap Paul, a veterinary surgeon, and William Barger, an orthopedic surgeon, began developing a surgical system based on IBM’s Puma arm to develop a robot to be used in hip replacement surgery. The Puma system was an early robotic interface, which enabled more precise preoperative planning in order to best match a femur with a prosthesis. The collaboration yielded ROBODOC (Integrated Surgical Systems, Sacramento, CA), a device that improved the ability to core out the femoral shaft from 75 % (standard human accuracy) to 96 % (Fig. 1.3).



Fig. 1.2
Puma 200 robot. With permission from M.L. Lorentziadis A Short History of the Invasion of Robots in Surgery. Hellenic Journal of Surgery (2014) 86:3, 117–121. © Springer

Fig. 1.3
ROBODOC surgical system. With permission from M.L. Lorentziadis A Short History of the Invasion of Robots in Surgery. Hellenic Journal of Surgery (2014) 86:3, 117–121. © Springer
In London, surgeon John Wickham, MD, and Brian Davies, PhD, used a system similar to ROBODOC (PROBOT) in its coring abilities, to perform a transurethral resection of the prostate. This was aided by a mechanically constrained ring in order to enhance safety and ensure precise movements of the robotic arm (Fig. 1.4).
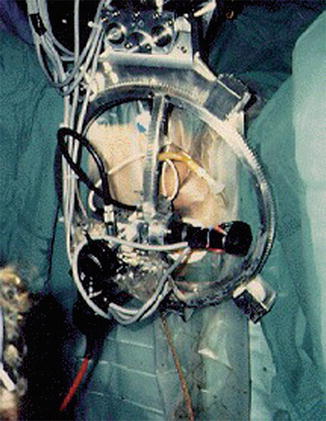

Fig. 1.4
PROBOT surgical robotic system (from http://www.imperial.ac.uk/ mechatronicsinmedicine/projects/probot/index.html). With permission from Ilya A. Volfson, Jeffrey A. Stock. History of Robotic Surgery. In: Jeffrey A. Stock MD, Michael P. Esposito MD, Vincent J. Lanteri MD, David M. Albala MD, edds. Current Clinical Urology: Urologic Robotic Surgery. Springer, New York 2008: pp 3–25. © Springer
Research is continued by the US military to develop a medical application for the use of remote telepresence surgery. In 1992, Risk Satava and Don Jenkins, working with Defense Advanced Research Projects Agency (DARPA), developed the MEDFAST (medical forward advanced surgical treatment) application. The goal was to maneuver the vehicle, a Bradley Fighting Vehicle (Fig. 1.5), onto the battlefield where injured soldiers could be treated by a field medic and surgery could be performed using the robotic arms mounted inside, while surgeons were stationed at a MASH (Mobile Advanced Surgical Hospital) unit. In 1996, a successful demonstration over a 5 km distance provided a proof of principle, though ultimately this system never took hold, mostly due to a shift from open battlefield grounds to more urban environments in which this model was not feasible.


Fig. 1.5
Bradley Fighting Vehicle. With permission from M.L. Lorentziadis A Short History of the Invasion of Robots in Surgery. Hellenic Journal of Surgery (2014) 86:3, 117–121. © Springer
Despite the success of ROBODOC, because of a prolonged approval process through the FDA, the first true mainstream application of the surgical robot was in the use of laparoscopy. Yulun Wang, a PhD working with DARPA, developed the AESOP system (Automated Endoscopic System for Optimal Positioning) (Computer Motion Inc., Santa Barbara, California). This was developed in order to provide a robotic first assist for the positioning and use of the laparoscopic camera (Fig. 1.6).


Fig. 1.6
AESOP (Automated Endoscopic System for Optimal Positioning). With permission from M.L. Lorentziadis A Short History of the Invasion of Robots in Surgery. Hellenic Journal of Surgery (2014) 86:3, 117–121. © Springer
At the same time as AESOP was becoming more prevalent, Frederick Moll, MD, licensed Green’s telepresence surgery device and formed Intuitive Surgical Inc. After some redesigning, the da Vinci system was born (Fig. 1.7). In April 1997, this system was used to perform the first true robotic surgery in Brussels by Drs. Himpens and Cardiere. In 1998, the da Vinci system was used to perform the first robotic valve surgery [4]. Soon after this, Computer Motion Inc. launched their system, ZEUS (Fig. 1.8). This system was similar to the da Vinci system in that robotic manipulator arms performed the surgery; however, the user interface was different in that it provided an ergonomically enhanced interface and an enhanced two-dimensional display. Initially the ZEUS system did not provide wristed instruments, although these were later introduced. This was used for the first full endoscopic robotic procedure, a fallopian tube reanastomosis.
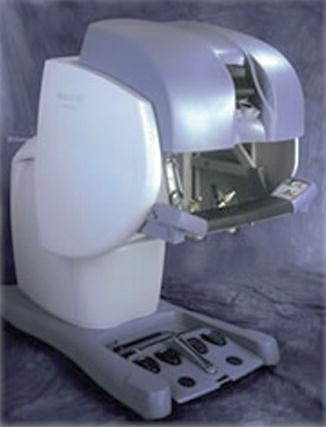

Fig. 1.7
da Vinci surgical system console. With permission from Ilya A. Volfson, Jeffrey A. Stock. History of Robotic Surgery. In: Jeffrey A. Stock MD, Michael P. Esposito MD, Vincent J. Lanteri MD, David M. Albala MD, edds. Current Clinical Urology: Urologic Robotic Surgery. Springer, New York 2008: pp 3–25. © Springer

Fig. 1.8
ZEUS Robotic Surgical System. With permission from Faust RA, Kant AJ, Lorincz A et al. Robotic endoscopic surgery in a porcine model of the infant neck. J Robotic Surg 2007;1:75–83
Finally, in 2003, Intuitive Surgical Inc. bought Computer Motion Inc. and the rest, as they say, “is history.” The da Vinci robotic surgical system was introduced to the market in 1999. At that time, it included a three-dimensional vision system, three arms, and the EndoWrist® technology. In 2003, its first major upgrade included the addition of a fourth arm to allow for enhanced manipulation and retraction. In 2006, the “S” system was released offering a high-definition vision and a multi-image display. In 2009, the “Si” system was released offering dual console capacity for enhanced training and collaboration as well as another enhancement of the visual system. Finally, this year (2014), Intuitive launched the “Xi” system which allowed for improved use and multi-quadrant surgery, upgrading the technology of its optics systems and using technology to improve the process of arm positioning and port placement. In addition, Intuitive Surgical Inc. introduced a fluorescence imaging system, Firefly™, to allow for direct visualization of tissue perfusion as well as the addition of energy and stapling tools married to the armature. The various da Vinci systems will be more fully discussed later in this chapter.
1.1 Current Applications
Although the first robotic-assisted coronary artery bypass was performed in Germany in 1998 [5], the Food and Drug Administration (FDA) only granted approval for selected robotic-assisted laparoscopic procedures in the Unites States in 2000. Since then, applications for robotic-assisted surgery have expanded exponentially. This section reviews some of the current applications and surgical procedures for which robotic assistance has been utilized over the past decade and a half.
1.1.1 Urology
1.1.1.1 Robotic Radical Prostatectomy
Prostate cancer is the second leading cause of cancer in men in the USA and worldwide. Radical prostatectomy is the standard surgical management for patients with localized disease; however, it is associated with high morbidity rates. Laparoscopic radical prostatectomy was introduced in 1997 by Schuessler et al. with the goal of reducing the morbidity associated with the open operation [6]. However, the technical challenges of laparoscopic radical prostatectomy proved to be difficult for the average urologist with limited laparoscopic experience and limited case volumes, and widespread adoption of the laparoscopic approach was not seen.
The introduction of the da Vinci® Surgical System paved the way for minimally invasive prostatectomy. Since the initial report by Menon et al. in 2002 [7] describing robotic prostatectomy utilizing the da Vinci® system, robotic prostatectomy has become the most widely performed robotic surgical procedure in the world. The robotic technique offers the advantages of improved ergonomics, easier laparoscopic suturing, and shorter learning curves. Following US FDA approval for the use of the da Vinci® system for radical prostatectomy in 2001, there was a 955 % increase in the number of robotic prostatectomies performed in the USA between 2003 and 2009 [8], with as many as 90 % of radical prostatectomies in the USA being performed robotically [9].
Interestingly, widespread adoption of robotic radical prostatectomy occurred in the early 2000s despite the lack of any convincing evidence demonstrating a clear benefit. It was not until 2012 when Trinh et al. demonstrated improved perioperative outcomes for patients undergoing robotic prostatectomy compared with open or laparoscopic prostatectomy, with fewer intraoperative and postoperative complications, decreased likelihood of requiring blood transfusion, and shorter length of stay [10]. Interestingly, despite the robot’s overwhelming prevalence, well-powered, randomized controlled trials demonstrating superior oncologic and functional (urinary continence and sexual potency) outcomes with the use of robotic-assisted prostatectomy are still lacking. Nevertheless, it is still considered by most to be the current gold standard in the surgical management of prostate cancer.
1.1.1.2 Robotic Partial Nephrectomy
In 2014, there will be an estimated 64,000 new cases of kidney cancer diagnosed and 13,860 deaths related to kidney cancer in the USA [11]. Given that radical nephrectomy has been shown to be an independent risk factor for new-onset chronic renal failure [12], there has been a trend in recent years toward performing partial nephrectomy when technically feasible. For small (<4 cm) early stage cancers, long-term cancer-free survival rates are comparable to those with radical nephrectomy; the incidence of local recurrence for partial nephrectomy is estimated at 0–10 % and is 0–3 % for tumors less than 4 cm [13].
While most would consider open partial nephrectomy the gold standard for nephron-sparing surgery, the procedure is associated with significant morbidity related to the muscle-cutting flank incision, namely, a high rate of hernia, pain, and paresthesia. In an effort to reduce the morbidity associated with open partial nephrectomy, laparoscopic partial nephrectomy was introduced and began to be more widely utilized in the late 1990s and early 2000s.
While laparoscopic partial nephrectomy is associated with shorter length of stay, decreased operative blood loss, and fewer wound-related complications, it is also a very technically challenging procedure with prolonged learning curves and has not quite gained the widespread acceptance that some thought it would. In fact, an unintended consequence of the push for more minimally invasive procedures for renal cancer was the resurgence in the number of radical nephrectomies being performed, as many viewed laparoscopic radical nephrectomy as a less technically challenging procedure than partial nephrectomy that still imparted the benefits of minimally invasive surgery (MIS) [14].
Robot-assisted partial nephrectomy has now largely replaced the traditional laparoscopic approach to partial nephrectomy, as it preserves the advantages of MIS, while at the same time being more technically feasible than laparoscopic partial nephrectomy, with a shorter learning curve. A number of case series have demonstrated equivalent early oncologic outcomes with robot-assisted partial nephrectomy compared with the traditional laparoscopic approach [15, 16]. A recent study utilizing data from the American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) database demonstrated that, when compared with the open approach, minimally invasive (including both laparoscopic and robot-assisted) partial nephrectomy resulted in shorter length of stay, fewer blood transfusions, and fewer complications, including pneumonia, wound infection, sepsis, and acute kidney injury requiring dialysis [17].
1.1.1.3 Robotic Cystectomy
Radical cystectomy is the standard surgical procedure for node-positive or muscle-invasive bladder cancer, but it can be associated with morbidity rates of up to 50 % and mortality rates as high as 5 % [18]. Laparoscopic cystectomy was first introduced in 1992 with the primary goal of reducing the morbidity rates seen with the open procedure and decreasing length of stay. While laparoscopic cystectomy has been shown to be associated with less morbidity, fewer blood transfusions, less postoperative pain, and shorter length of stay when compared with open cystectomy [19], it suffers from the same drawbacks as laparoscopic partial nephrectomy, namely, extreme technical challenges and a long learning curve.
The first robotic-assisted radical cystectomy was performed in 2003 [20]. The robotic approach has since been seen as a means to potentially overcome the technical challenges of laparoscopic cystectomy. However, the procedure is still in its relative infancy, and there is a paucity of level I data comparing robotic-assisted cystectomy with open and laparoscopic cystectomy. A prospective, randomized controlled trial published by Bochner that randomized patients to open vs. robotic-assisted radical nephrectomy demonstrated similar perioperative complication rates and length of hospital stay. In this study, the robotic-assisted approach was associated with less intraoperative blood loss, but had an average operative time 127 min longer than the open technique; the authors concluded that this argued against the benefit of robotic-assisted cystectomy in terms of minimizing perioperative morbidity [21]. A recent non-randomized, retrospective study comparing open and robotic-assisted radical cystectomy showed no difference in surgical margin status and lymph node yield, though there were less need for transfusion and a 20 % reduction in length of stay in the robotic group [22].
While preliminary reports of robotic-assisted cystectomy are encouraging, long-term functional and oncologic results are still widely unknown. Certainly, the procedure seems to hold promise for the future, though further study is clearly needed before it gains the acceptance that robotic prostatectomy has seen.
1.1.2 Cardiothoracic Surgery
One of the earliest applications of robotic surgery was in cardiothoracic surgery, with a robotic-assisted mitral valve repair being performed by Carpentier in 1998 [23]. Robotic-assisted cardiac procedures currently being performed include mitral valve repair and replacement, coronary artery bypass grafting, closure of atrial septal defects, implantation of pacing leads, and resection of intracardiac tumors. Robotic mitral valve surgery is probably the most widely used application of robotic assistance in cardiac surgery and has been shown to be associated with decreased rates of postoperative atrial fibrillation and pulmonary effusion, as well as decreased length of stay, while maintaining similar perioperative complication rates to those seen with open procedures [24, 25]. The complexity of the procedure and longer operative times are felt by its proponents to be offset by the shorter length of stay and other potential benefits of the minimally invasive approach to mitral valve surgery.
Robotic-assisted thoracic surgical procedures currently being performed include pulmonary resection and mediastinal lymphadenectomy for lung cancer, thymectomy, and removal of mediastinal tumors. The adoption of robotic lobectomy has gained significant momentum in recent years, despite the majority of trials showing equivocal results. A recent national database review did demonstrate that robotic pulmonary resections were associated with significant reductions in mortality, length of stay, and overall complication rates compared with open thoracotomy [26]. Robotic-assisted esophagectomy has also been recently introduced, with early reports suggesting similar outcomes to those seen with laparoscopic and open esophagectomy [27].
Despite being one of the pioneering specialties in the development and implementation of robotic-assisted surgical technology, it is still somewhat unclear as to whether or not there is a distinct advantage afforded to patients undergoing robotic-assisted cardiothoracic procedures compared with those undergoing open procedures. Given prolonged operative times and the increased cost associated with the use of robotic technology, combined with largely equivocal results in the literature thus far, further evidence and results from well-powered, randomized controlled trials are needed before robotic-assisted cardiothoracic surgery becomes a standard of care.
1.1.3 Colon and Rectal Surgery
While laparoscopic colon and rectal resection for benign disease has been performed for some time now, laparoscopic resection for malignant disease did not become an accepted means of surgical treatment until the results of the Clinical Outcomes of Surgical Therapy (COST) trial were published in 2004, demonstrating equivalent oncologic outcomes between laparoscopic and open colectomy [28]. The use of laparoscopy for colorectal resection, however, is fraught with a number of difficulties, including the need to work in multiple quadrants, lack of fixation of the transverse and sigmoid colon, and difficult access to the pelvis. The addition of robotic technology to traditional laparoscopic techniques has sought to overcome the difficulty of operating in the pelvis but augments the challenge of operating in different quadrants and provides zero tactile feedback.
Colon and rectal surgical procedures that have been described using a robotic-assisted approach include segmental and total abdominal colectomy, low anterior resection, abdominoperineal resection, restorative proctocolectomy, and rectopexy (with and without sigmoid colectomy) for rectal prolapse. The number of publications describing techniques and outcomes for robotic-assisted colon and rectal surgery continues to grow exponentially, yet a great deal of controversy still remains regarding the exact role of robotics in colon and rectal surgery.
While a number of studies have shown robotic colon and rectal surgery to be safe and technically feasible [29–31], two of the major deterrents to its more widespread adoption, similar to that seen in other specialties, are longer operating times (including operating room setup and breakdown) and greater cost. In fact, a recent study comparing robotic-assisted and conventional laparoscopic right colectomy [32] found that while there were equivalent morbidity rates, lymph node retrieval, blood loss, and length of stay, the robotic group clearly had significantly longer operative times and increased cost, arguing that robotic surgery for right colectomy may only be disadvantageous.
An area where some feel that longer operative times and increased cost may be justified is the in the realm of rectal cancer surgery. Total mesorectal excision (TME) has become the gold standard in surgery for rectal cancer [33], but this can be a challenging procedure to perform laparoscopically, particularly in obese patients and those with a narrow pelvis. The quest for a complete, intact TME specimen, coupled with the desire to minimize pelvic autonomic nerve injury and postoperative genitourinary dysfunction, has raised the bar in terms of the need for surgical precision when operating in the pelvis. Proponents of robotic-assisted rectal resection argue that improved outcomes in terms of oncologic and functional outcome may be achievable due to more precise identification of anatomic planes and neurovascular structures, aided by the magnified, three-dimensional high-definition images provided by the robotic platform as well as improved tissue handling facilitated by wristed instruments.
A body of evidence is growing in the literature indicating that robotic surgery may be equivalent to open and conventional laparoscopy for rectal cancer. De Souza et al. published a comparative series of robotic vs. open TME that demonstrated similar perioperative morbidity, length of stay, and wound infection rates between the two groups, while the robotic group has less blood loss but longer operative times [34]. A number of other studies have demonstrated similar and, in some cases, better complication rates and short- to intermediate-term oncologic results for robotic-assisted rectal resection when compared to open and conventional laparoscopic rectal resection [35–37]. A recent meta-analysis comparing robotic-assisted and laparoscopic TME published by Xiong showed that robotic TME is safe and feasible and the short- and medium-term oncologic and functional outcomes are equivalent to laparoscopic TME [38].
As previously discussed, one of the proposed benefits of robotic-assisted surgery for rectal cancer is the potential for better visualization of the neurovascular bundles in the pelvis and reduced rates of genitourinary dysfunction. However, data supporting this fact had been lacking until recently, when Kim et al., in 2012, reported that robotic TME, in their experience, was associated with earlier recovery of normal voiding and sexual function compared to patients undergoing conventional laparoscopic TME [39]; others have since reported similar findings [40, 41].
The issues of increased cost and operative time have been major deterrents to the more widespread use of robotic TME. However, Byrn et al. have actually shown that the issues of longer operative time and increased cost may become offset with experience and increased surgical volume. They compared their first 43 robotic-assisted rectal resections with their next 42 robotic-assisted rectal resections and found that, despite the latter group having a higher body mass index, shorter operative times were seen; direct hospital costs were also lower in the latter group, though this did not reach statistical significance [42].
The future of robotic colon and rectal surgery remains somewhat unclear. Many continue to raise the question as to whether the potential benefits are offset by the increased cost and operative time. Additionally, data regarding improved functional outcome for robotic-assisted rectal surgery are just beginning to appear in the literature, and we are still awaiting long-term results with regard to oncologic outcomes. It has been proposed that with the further development and advancement of single-site robotic technology and techniques, there may be further increased interest in advancing robotic-assisted colon and rectal surgery in coming years [43].
1.1.4 General Surgery
Robotic applications in general surgery are widespread and include gynecology [44–51], cholecystectomy, bariatric surgery, anti-reflux surgery, hepatobiliary surgery, gastrectomy, and splenectomy. Conclusions regarding any clear benefit of the robotic-assisted approach for these procedures, as well as their safety and efficacy, are limited due to a lack of well-powered, prospective randomized clinical trials. Nonetheless, as robotic surgery becomes more accepted, newer applications for this technology continue to be explored within the realm of general surgery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







