(1)
Department of Vascular Surgery, Evangelisches Krankenhaus Königin Elisabeth Herzberge, Berlin, Germany
19.1 Introduction
Stenosis, thrombosis, and aneurysmatic dilatation are the most frequent postoperative complications in vascular access surgery. Microscopic analysis of resected specimens or small biopsies can help explain the exact etiology of these complications (Janzen 2005, 2010).
The surgeon should communicate whether the histopathological specimen consists of:
The wall of the native vein.
The wall of the arterialized vein which has been dissected for the creation of the original vascular access.
The wall of a vein which had not been touched previously for its in situ arterialization.
The hypertrophic wall of a vein at a puncture site.
The hypertrophic and dilated wall of a vein which has not been exposed during former surgery (Krönung 2008).
Exact specifications of the site from which the specimen has been taken, type of prosthesis, and the patient’s history may help the vascular pathologist find the diagnosis. As for light microscopy, the arterialized vein of a fistula shows an irregular fibrosis of the tunica media, a newly-formed respectively double internal elastic membrane, and hypertrophy/hyperplasia of the smooth muscle cells of the tunica media (Figs. 19.1 and 19.2). In rare cases myocytic hyperplasia is a precursor of fibromuscular dysplasia in the tunica media.
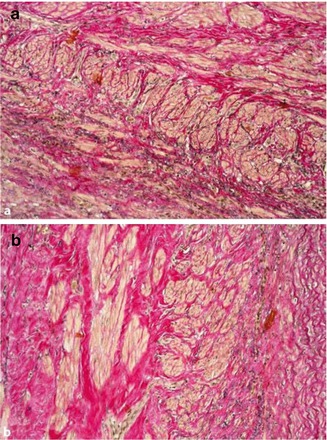
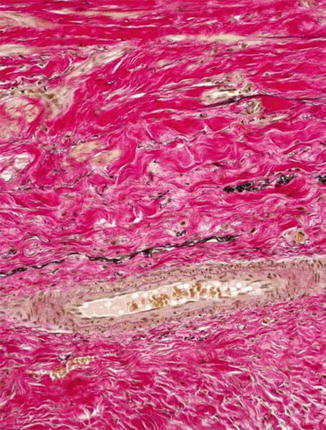

Fig. 19.1
Arterialized vein of a cephalic fistula with myocytic hyperplasia/hypertrophy. (a) With irregular media fibrosis. (b) Elastica van Gieson (EVG) stain, ×200

Fig. 19.2
Newly-formed internal elastic membrane (black in the middle) in arterialized fistula vein, EVG stain, ×200
19.2 Stenosis
The histomorphologic spectrum of the underlying changes of fistulas is heterogenous. Non-atherosclerotic causes dominate. In clinical jargon, stenosis is generally referred to as neointima. However, an exact histopathologic analysis shows a far more detailed picture. The intraoperative finding of an “inflammatory” shrinking (Fig. 19.3), for instance, does not describe a genuine inflammatory process, but mostly an intimal hyperplasia. This can be divided into three subtypes – neointimal, fibrotic, and combined intimal hyperplasia (Figs. 19.4, 19.5, and 19.6) (Janzen and Mickley 2006).
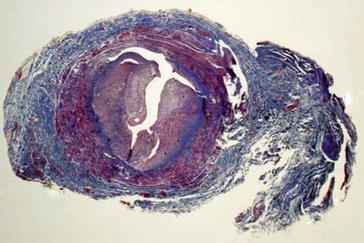
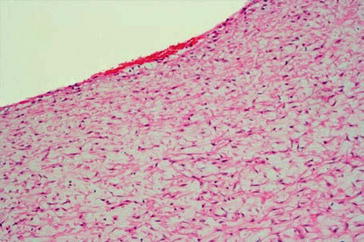
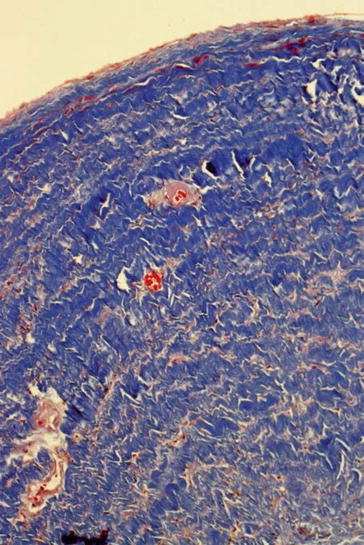
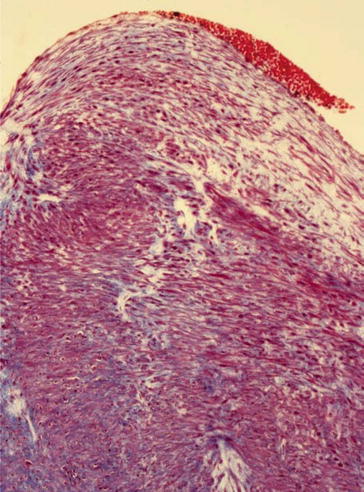

Fig. 19.3
Shrunk fistula vein, MTC stain, ×200

Fig. 19.4
Neointimal type of subendothelial intimal hyperplasia, H&E stain, ×100

Fig. 19.5
Fibrotic type of subendothelial intimal hyperplasia, MTC stain, ×100

Fig. 19.6
Combined type of a subendothelial intimal hyperplasia (neointimal-fibrotic mixed type), EVG stain, ×100
The pathologic alterations are located in the subendothelial stratum of the tunica intima. Therefore we prefer subendothelial intimal hyperplasia (SIH) to intimal hyperplasia (IH). SIH is one of the main causes for stenosis formation.
The neointimal SIH consists of myofibroblasts arranged in a star-shaped pattern, fibroblasts, fibrocytes, and a pale eosinophilic matrix. Overlying endothelium shows enlarged nuclei as a sign of increased metabolic activity. Their development mainly seems to be favored by local hemodynamic factors such as altered wall shear stress and oscillating forces (Haruguchi and Teraoka 2003; Sivanesan et al. 1999). Specific immune markers (calponin, actin, smooth muscle actin, desmin, vimentin, etc.) help distinguish between the different cell types of the neointima (Fig. 19.7). In IH increased cell proliferation and apoptosis (oncoprotein bcl-2), raised growth factors, an overexpression of Bax proteins/Bax mRNA, and a significant correlation between the extent of a stenosis and bcl-2-positive areas can be seen (Rekhter et al. 1993; Hayakawa et al. 1999; Weiss et al. 2001). Furthermore, arterial as well as venous forms of IH have been postulated. Kim et al. (2004) assumed a different biological behavior of vascular smooth muscle cells. Others claim that the following criteria have to be fulfilled for a venous IH:
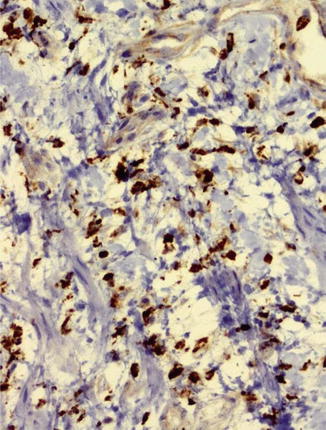

Fig. 19.7
Neointima with calponin expression of myofibroblasts, ×200
In my experience there are light microscopically identical pictures of arterial and venous SIH (Fig. 19.8). The neointimal SIH can easily be distinguished from the fibrotic SIH. There are, however, also smooth transitions between these two entities so that most likely neointimal SIH is a preliminary stage of fibrotic SIH. The latter is histomorphologically characterized by a lower cell count and the presence of collagen subtypes I, III, and IV. Remarkably there is no atherosclerosis whatsoever in the shrunken fistula veins (Janzen and Mickley 2006, 2007).
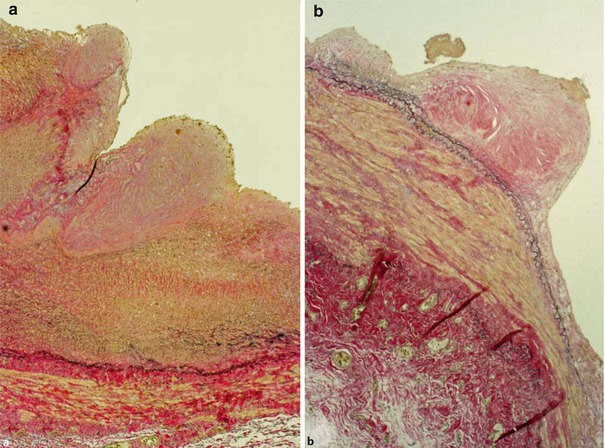

Fig. 19.8
Fibrotic subendothelial intima hyperplasia in the (a) arterial and (b) venous anastomosis, EVG stain, ×40
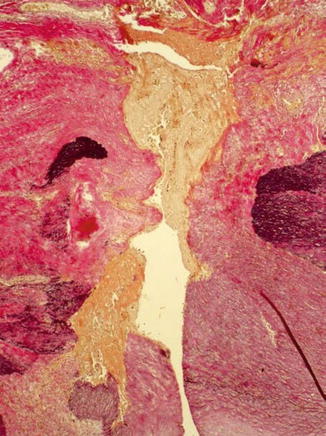
19.3 Aneurysmatic Dilatation
Several factors influence aneurysmatic dilatation. Repeated punctures for hemodialysis weaken the walls of fistulas or grafts (Figs. 19.9, 19.10, 19.11, 19.12, and 19.13), but the intravascular fistula pressure is also an important factor. Should the internal elastic lamina membrane rupture due to an increase in pressure, this event will accelerate the formation of an aneurysm (Fig. 19.14). Puncture trauma also causes false aneurysms (pseudoaneurysms) of the native fistula or the graft. There blood collects in the neighboring fibrolipomatous soft tissue (Figs. 19.15, 19.16, and 19.17) (Janzen et al. 2007).