THE MAJOR HISTOCOMPATIBILITY COMPLEX
Human MHC Gene Cluster
The human MHC comprises about 3.6 Mb DNA (0.1% of the genome) located on chromosome 6p21.31. The MHC is the most gene-dense region of the human genome comprising more than 220 genes. The average gene density over the entire MHC region is one gene per 16 kilobases (kb). Only 50% of the genes in MHC region appear to be expressed, and the remainder are unexpressed pseudogenes. One possible explanation for maintaining such high levels of pseudogenes could be that they are involved in generating new alleles by gene conversion, a phenomenon that has been observed at other human immune loci. About 40% of the expressed genes have immune system function.
The human MHC has been divided physically into three regions: class I (telomeric), class II (centromeric), and class III (central region, see
Chapter 2,
Fig. 2.1). The HLA class I cluster comprises three classic class I genes (HLA-A, -B, and -C), three nonclassic class I genes (HLA-E, -F, and -G), two class I-like genes (MHC class I-related chain A [MICA] and MHC class I-related chain A [MICB]), and several pseudogenes. The classic class I genes are constitutively expressed by all nucleated cells and control the activation and function of cytotoxic T lymphocytes. The expression of nonclassic class I antigens is restricted to specific tissues, whereas the class I-like genes are expressed under some physiologic stress conditions. The products of both nonclassic and class I-like genes serve as ligands to receptors that control the function of natural killer cells.
The HLA class II cluster comprises classic class II genes (HLA-DR, -DP, and -DQ), nonclassic class II genes (HLA-DM and -DO), and several pseudogenes. The HLA-DR region contains one functional gene for the α chain (DRA) but has one or two functional genes for the β chain, depending on the HLA-DR type. All HLA-DR types have the DRB1 gene, and some contain an additional functional DRB gene, DRB3, DRB4, or DRB5, which forms a second cell surface heterodimer with the DRA-encoded α chain. HLA class II molecules are constitutively expressed by antigen-presenting cells (dendritic, macrophage, and monocyte cells) and B lymphocytes, but these antigens can be induced on activated T cells and endothelial cells, including the glomerular endothelium, renal tubular cells, and capillaries. The nonclassic class II genes are not expressed on the cell surface but form heterotetrameric complexes involved in peptide exchange and loading onto classic class II molecules. The class III region comprises genes that play in critical immune function such as those encoding tumor necrosis factors, complement proteins, and heat shock proteins.
Structure and Function of HLA Molecules
Although MHC molecules are important barriers to transplantation, their primary function is to provide protection against pathogens. The HLA molecules evolved with an appropriate structure to perform this specialized antigen presentation function in an effective manner. Although class I and class II HLA molecules are encoded by different genes and comprise distinct subunit structures, they are remarkably similar in their three-dimensional crystallographic structures (see front cover).
The class I antigens (HLA-A, -B, and -C) consist of an α heavy chain of 45 kDa with three globular external domains (α1, α2, and α3), a transmembrane region, and an intracellular domain. The structure is stabilized by a non-MHC encoded β
2-microglobulin (located in chromosome 15) associated with the α3 domain. The class II antigens (HLA-DR, -DQ, and -DP) consist of two noncovalently linked chains: an α-chain of 35 kDa (encoded by DRA, DQA1, or DPA1) and a β chain of 31 kDa (encoded by DRB1, DRB3, DRB4, DRB5, DQB1, or DPB1). Both chains are transmembrane with two globular extracellular domains. The αl and α2 domains of class I molecules fold together into a single structure consisting of two segmented α
1 helices lying on a sheet of eight antiparallel β strands. The folding of the αl and α2 domains creates a long cleft or groove facing away from the cell, in which peptides bind. Similarly, the membrane distal αl and β1 domains of class II molecules form the peptidebinding cleft. The class I and class II molecules differ with regard to the ends of the groove that are closed in class I and open in class II molecules, permitting longer peptides to be accommodated on class II molecules. The HLA antigens (self) with their loaded peptides (nonself) are exposed to T cells, which recognize these compound structures (self + nonself) through their T-cell receptors and trigger immune activation against the foreign antigens (See
Chapter 2).
The Nature of HLA Polymorphism
The classic class I and class II genes encode HLA molecules, the most polymorphic proteins known to date in humans. Early studies using serologic typing methods identified an unprecedented number of HLA alleles at each locus. DNA sequencing revealed an even more extensive polymorphism because the serologically defined antigens included multiple allelic variants that could differ by a single nucleotide substitution. The differences among HLA proteins are localized in the antigen-binding domain, particularly enriched in positions that interact with antigenic peptides or the T-cell receptor. Class I polymorphisms are predominantly found in the first 180 amino acids of the heavy chain, and
class II polymorphisms are found in the first 90 to 95 amino acids of the α or β chains, or both. This extreme polymorphism is thought to be driven and maintained by the long-standing battle for supremacy between the immune system and infectious pathogens.
Most amino acid substitutions are shared by more than one HLA molecule and thus demonstrate a patchwork pattern of sequence polymorphism. Antigenic determinants with unique sequence motifs (also called epitopes) are known as private specificities. Some antigenic determinants are shared by many HLA antigens. These are called public specificities. The Bw4 and Bw6 specificities are good examples of public antigens. All HLA-B antigens express either Bw4 or Bw6. The antigenic determinant that defines these specificities is affected by amino acids in positions 80 and 83 of the class I molecule sequences located in the exposed part of the α1 helix. Class I molecules with arginine at position 83 and threonine or isoleucine at position 80 are recognized by anti-Bw4 antisera and include the HLA-B13, -B17, -B27, -B37, -B38, -B44, -B47, -B49, -B51, -B52, -B53, -B57, -B58, -B59, -B63, and -B77 antigens. The HLA-A23, -A24, -A25, and -A32 antigens also have the characteristic arginine at position 83 and react with anti-Bw4 antibodies. All other B-locus antigens have glycine at position 83 and asparagine at position 80 and react with anti-Bw6 antibodies. A consequence of the patchwork pattern of HLA polymorphism is that an antibody generated against a particular antigen may react to a number of HLA antigens that share the same sequence motifs, a problem referred to as crossreactivity. For instance, a patient’s serum carrying anti-HLA-A2 antibodies may react to HLA-A2, as well as -A68, -A69, -B57, and -B58, because these antigens share amino acid sequence motifs with HLA-A2.
HLA Nomenclature
One of the most notable features of the HLA system is the remarkable degree of polymorphism exhibited by its gene components. Even when we limit the discussion to the products of the HLA-A, -B, and -DR loci, which are most commonly encountered in clinical kidney transplantation, there are 88 recognized antigens (defined by antibodies), encoded by more than 2200 distinct alleles (
Table 3.1), and the number of new alleles is still increasing. Obviously, keeping track of this diversity requires a specialized nomenclature. The HLA antigens were identified and characterized over a 50-year period beginning with the discovery of the MAC (now HLA-A2) antigen by Dausset in Paris in 1958. A series of international workshops, beginning in 1964 and held about every 4 years until 1987, established a nomenclature for the HLA antigens, naming unique antigens in the sequence in which they were officially recognized: Al, A2, A3, Bw4, B5, Bw6, B7, B8, and so on. The antigens were identified using antisera obtained primarily from multiparous women. As the field evolved, new antisera were discovered that could “split” some HLA antigens into narrower specificities. HLA-A9 was split into HLA-A23 and -A24, and HLA-A10 was split into HLA-A25, -A26, -A34, and -A66, for example.
Table 3.1 lists the broad parent antigens for splits in parentheses.
The already complicated HLA nomenclature became more complex when DNA-based typing technologies for HLA were developed in the mid-1980s. To accommodate the growing numbers of alleles that could be identified by their unique nucleotide sequences within the antigen designations, the established serologic nomenclature was modified to associate alleles with antigens whenever possible, and four-digit designations were developed in which the antigen designation makes up the first two digits and the sequential allele designation makes up the third and fourth digits. The first allele for HLA-A1 is HLAA*0101, which includes the locus (
A), an asterisk (*) to indicate the typing was performed by DNA methods, the serologic antigen (
01), and the allele number
(
01). In cases in which the total number of coding variants exceeds 99, a second number series is used to extend the first one. For example, for the very large B*15 family of alleles, the B*95 series is used to code for additional B*15 alleles. Consequently the next B*15 allele named following B*1599 was B*9501. Likewise the A*92 series has been used as a second series for the A*02 allele family. The naming of HLA class II antigens is similar, even though two distinct polypeptides encoded by separate genes combine to form the antigen. The DR antigens are distinguished by their DR β
1 subunit; therefore, the first allele of DR1 is DRB1*0101.
There are some exceptions that may be confusing. The HLA-B14, -B15, -B40, and -DRB1*03 allele series include distinct antigens that are both immunogenic and antigenic. The HLA-B62 antigen, for example, is encoded by HLAB*1501, 1504, 1505, 1506, 1507, and many other B15 alleles, whereas HLA-B75 is encoded by HLA-B*1502, 1508, 1511, and so on. HLA-DRB1*0301 is HLA-DR17, whereas HLA-DRB1*0302 is HLA-DR18. The correlation between alleles and antigens is updated periodically in the
HLA Dictionary and in the series “Nomenclature for Factors of the HLA System” (see “
Selected Readings”).
Although the number of HLA antigens, alleles, and combinations is very large, the frequencies of individual antigens, alleles, and combinations in a given population vary considerably. The most common HLA antigen is A2, which is found in roughly 50% of individuals from populations around the world. About 96% of whites with European ancestry who express HLA-A2 have the HLA-A*0201 allele. Northern Chinese and many Hispanics who express HLA-A2 have the HLA-A*0206 allele. HLA-B8 is found in 30% of Scots, and the frequency declines as populations in Europe and more distant areas are analyzed, except in those areas that were colonized by the British (South Africa, India, Australia), where the frequency is higher. Thus, certain antigens and alleles are common, whereas others are very rare, and, in fact, no frequencies have been established yet for the majority of alleles because they have not been encountered or detected among donors and recipients. Some HLA antigens are racially limited. Thus, HLA-B54 is found almost exclusively in persons from Japan and nearby Asian countries. HLA-A36 is relatively common among blacks but is very rare in other populations.
The additional HLA polymorphism that has been revealed through the application of DNA technologies has provided interesting insights into the role of HLA in many autoimmune diseases, but its significance in clinical kidney transplantation remains to be seen. Allele differences between the donor and recipient of bone marrow transplants lead to graft-versus-host disease. However, extensive analysis of HLA allele-level mismatches among HLA antigenmatched kidney transplant recipients has revealed no substantial effect of allele-level HLA mismatches on graft survival rates.
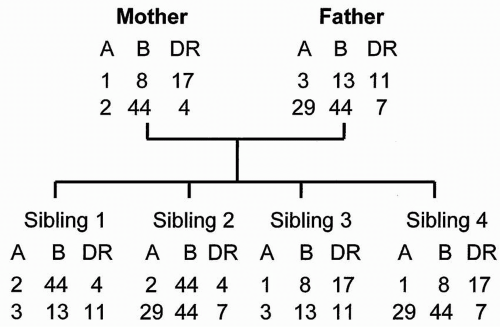
Family Segregation of HLA Haplotypes
Each parental chromosome 6 provides a haplotype or linked set of MHC genes to the offspring (
Fig. 3.1). Haplotypes are usually inherited intact from each parent, although crossover between the A and B locus occurs in about 2% of offspring, resulting in a recombination (and a new haplotype). The child carries one representative antigen from each of the class I and class II loci of each parent. A child is, by definition, a one-haplotype match to each parent unless recombination has occurred.
HLA haplotypes are inherited in a mendelian fashion. Statistically, there is a 25% chance that siblings share the same haplotypes (two-haplotype match), a 50% chance they share one haplotype (one-haplotype match), and a 25% chance that neither haplotype is the same (zero-haplotype match). Even in the case of
siblings who share both HLA haplotypes, 25% to 100% of other parental chromosomes may be different, and these other chromosomes include other “minor” histocompatibility antigens, which can also initiate rejection reactions.
Definition of Haplotypes and Phenotypes
Consider an individual with the following HLA profile or phenotype: Al, A24, B8, B44, DR4, DR15. From this phenotypic information alone, it is not possible to identify haplotypes because it is not known which antigens are linked on each chromosome. Consider another individual with the following HLA phenotype: Al, A3, B7, B8, DR4, DR12. If this second individual is the biologic parent, offspring, or sibling of the first individual, it becomes possible to identify a shared haplotype of the family as Al, B8, DR4. The first individual also has an unshared haplotype A24, B44, DR15, and the second individual an unshared haplotype A3, B7, DR12. These haplotypes should appear in the parents and other siblings. A kidney transplanted between these two individuals would be a one-haplotype matched graft, and the Al, B8, and DR4 antigens would be genotypically identical in the donor and recipient because they are encoded by the same inherited genes.
If these two individuals are not related, it is not possible to identify the haplotypes. Thus, in transplants from living unrelated or deceased donors, the haplotypes are unknown, and only the phenotypic identity of individual HLA antigens can be determined. The two individuals whose HLA phenotypes are listed would be called a
three-antigen match or a
three-antigen mismatch (see “
HLA Matches and Mismatches”). Sharing of minor histocompatibility antigens is serendipitous.
Linkage Disequilibrium
Although it is not possible to identify an individual’s haplotypes from the phenotypic HLA typing information alone, within racial or ethnic populations, certain HLA determinants are inherited together more often than would be expected by chance. For example, if HLA-A1 and HLA-B8 occur at gene frequencies of 16% and 10%, respectively, in a population, the probability of finding them together should be 1.6%. However, the actual occurrence rate of the HLA-A1-B8 combination is significantly above the predicted incidence (about
8%). This phenomenon represents the inheritance of haplotypes within racial groups. Existing data suggest that positive selection is operating on the haplotype and that the linked loci confer a particular selective advantage for the host.
HLA Matches and Mismatches
It is not always possible to identify two HLA specificities at each HLA locus. Consider the HLA phenotypes for the following two unrelated individuals:
1. A2, —; B27, B13; DR17, DR4
2. A2, A3; B8, B14; DR17, —
The absence of the second A-locus antigen in individual 1 and the second DR-locus antigen in individual 2 could result from a failure to identify the second antigen. More often, it reflects the inheritance of the same antigen (A2 and DR17 in these cases) from both parents (the individuals are homozygous at these loci). Among whites, the latter is usually the case. A kidney transplanted between these two individuals would be described as a one A and one DR match, but this terminology does not take into account homozygosity in the A and DR loci of individuals 1 and 2, respectively. If individual 1 were a donor for individual 2, it would be more informative to describe the combination as a zero A, two B, and one DR mismatch. If individual 2 were a donor for individual 1, the combination would be a one A, two B, and zero DR mismatch. Antigenic differences in the donor kidney are potential targets of rejection; therefore, the convention of counting the number of donor HLA antigens that are not shared by the recipient provides an estimate of the antigen dose.
Identical and Fraternal Twins
The differentiation between identical twins and two-haplotype-matched fraternal twins is important because the recipient of a transplant from an identical twin requires no immunosuppression. The procedure is immunologically equivalent to an autotransplantation. Two-haplotype-matched siblings, whether they are fraternal twins or not, differ in their minor histocompatibility antigens, and immunosuppression is required. Monozygotic, or identical, twins share a single placenta and amniotic sac at birth. However, such information may be unavailable or unreliable when the patient and donor are evaluated as adults. A variety of methods have been used to identify monozygotic twins, including skin grafting from the potential twin donor to the recipient (the graft would be rejected if the twins were fraternal). Today, several genetic polymorphisms can be exploited to determine identity at many genetic loci, providing a high degree of confidence that twins are identical. Extended blood groups include markers that are determined by many genes on different chromosomes. Analysis of short tandem repeats (STRs), which, as the name implies, are short nucleotide sequences that are repeated a variable number of times, provide a high probability of identifying differences between individuals. STRs are often used in monitoring engraftment of HLA-identical bone marrow transplants, so they are exquisite markers of individuality.