Fig. 5.1
The layered structure of gastric and colonic wall (Modified from Yamanaka [2]; m mucosa, sm submucosa, pm muscularis propria, ad adventitia, s/ss subserosa/serosa, ep lamina epithelialis, gl glands, tp tunica/lamina propria, mm muscularis mucosae, ic intermuscular connective tissue, 1–13 number of layers that can be identified)

Fig. 5.2
The layered structure of the oesophageal wall (Modified from Yamanaka [2]; m mucosa, sm submucosa, pm muscularis propria, ad adventitia, s/ss subserosa/serosa, ep lamina epithelialis, gl glands, tp tunica/lamina propria, mm muscularis mucosae, ic intermuscular connective tissue, 1–13 number of layers that can be identified)
5.3 Clinical Staging of Mucosal Neoplasms
Both T- and N-staging are important determinants of any therapy proposed, and data support the notion that endoscopic ultrasound is more reliable than CT in detecting lymph nodes. Conventional endoscopic ultrasound using frequencies of 7.5 (3.5–15) MHz allows for an excellent visualisation of affected surrounding structures and their involvement in the disease. However, with respect to endoscopic mucosal resection, it is necessary to allow for precise staging within the T1 category, i.e. T1M and T1sm and further within these categories in sm1/2/3, respectively.
5.3.1 T-Staging Using hrEUS
T-staging by hrEUS has a higher accuracy when compared to conventional endoscopic ultrasound (81 % vs. 56 %) in the upper gastrointestinal tract [3]. Several studies have reported on the rates of lymph node metastases with respect to invasion depth of the tumour. Simply, the deeper the tumour invades the layers of the gastrointestinal tract, the more likely metastases do occur [2–15]. Hence, the correct estimation of invasion depth of the tumour not only defines the lesion which could safely be resected endoscopically but also translates into the understanding of the risk for metastases and herewith the choice of the appropriate therapy.
The value of hrEUS to differentiate T1m and T1sm of early oesophageal neoplasias prior to therapy has been reported [5]: an accuracy of 73.5 % with a sensitivity of 62 % and specificity of 76.5 % have been found. In general, the accuracy of miniprobe ultrasonography has been shown to be around 80 % in the oesophagus, the stomach and the colon and rectum.
The correct attribution of the infiltration depth can further be improved, when the shape of the infiltrating process is considered.
Submucosal invasion was in 60 % of lesions present, when an “irregular narrowing” can be seen and even in 86 % of lesions with “budding sign” (defined as an infiltrating hypoechoic process of 2 mm width) [8]. The accuracy could be as high as 90 % for hrEUS [16]: a hypoechoic area limited to the mucosa or a fan-shaped hypoechoic area in the submucosal layer could be classified as EUS-m/sml (Fig. 5.3a), an arch-shaped hypoechoic area in the submucosal layer as EUS-sm2 (Fig. 5.3b) and an arch-shaped hypoechoic area spreading to the muscularis propria as EUS-ad (i.e. T2, Fig. 5.3c). The arch-shaped hypoechoic area distinguishes deeply sm2-/3-invasive lesions from mucosal/sml-microinvasive, indicating endoscopic resection is inappropriate.
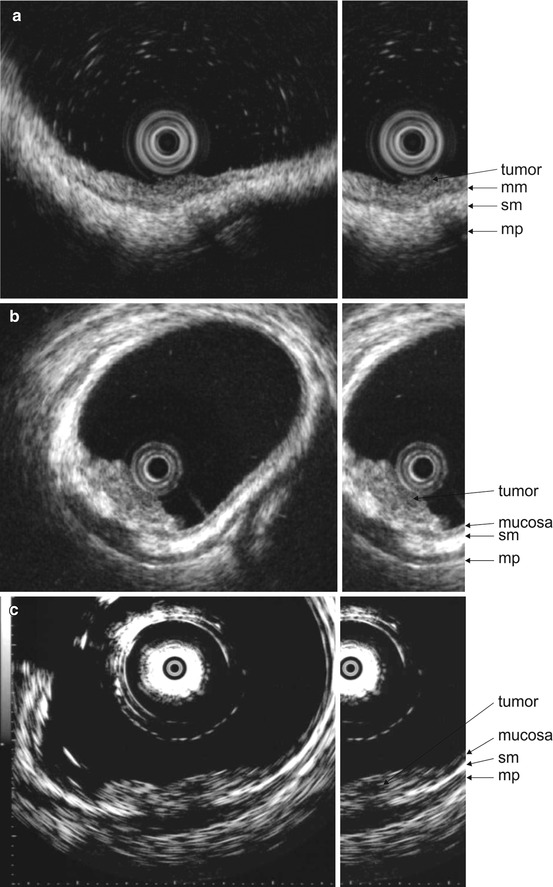

Fig. 5.3
(a) Lateral spreading tumour of the sigmoid, M in high-resolution endosonography. Abbreviations: sm, submucosa; mm, muscularis mucosae; mp, muscularis propria. (b) Lesion 0-Is of the rectum, sm2 in high-resolution endosonography. Abbreviations: sm, submucosa; mp, muscularis propria. (c) Lesion 0-IIa + IIc at the rectum, T2 in high-resolution endosonography. Abbreviations: sm, submucosa; mp, muscularis propria
5.3.2 N-Staging Using hrEUS and Accuracy
Endoscopic ultrasound has proven to be superior over CT in early oesophageal cancer [11]. The accuracy of miniprobe ultrasonography in N-staging has been shown to be between 56 and 87 % in the gastrointestinal tract, whilst sensitivity rates were somewhat lower (Table 5.1). Therefore, conventional endoscopic ultrasound is more adequate to address N-stage of a given lesion.
5.4 Limitations of High-Resolution Endosonography
Endosonography is depending on good transmission of the acoustic signal. The transmission can easily be disturbed by small gas bubbles or even mucus especially in hrEUS. In addition, the anatomical situation may sometimes not allow for a perpendicular image plane and a depiction in the focal zone of the probe. A lesser exact estimation of invasion depth might occur in protruding or pedunculated lesions, large lesions or at the gastroesophageal junction. The accountable anatomic peculiarity may result in misinterpreting of the stage and an error probability of up to 27 % with 19 % of lesions being overstaged [5]. hrEUS may be less accurate with tumour size exceeding 2 cm, submucosal invasion and ulceration [20–22].
Also, when hrEUS is compared to high-resolution endoscopy, there seems to be a slight albeit not significant advantage of endoscopy over endosonography. An 80–90 % accuracy for endoscopy compared to 80–85 % accuracy for hrEUS has been reported in early oesophageal cancer [9, 23]. Therefore, endoscopy alone might be adequate to decide on a therapeutic approach [24], especially when high-resolution or optical or digital frequency selection is possible.
5.5 Cases: High-Resolution Endosonography and Endoscopic Analysis of Mucosal Neoplasms
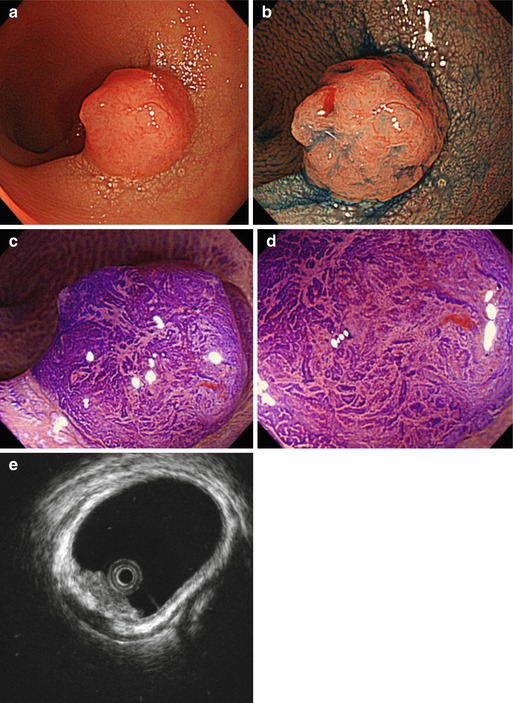
Case 1: Small Rectal Lesion 0-Is
On screening endoscopy, a small 10 mm polyp 0-Is with irregular, in part depressed surface was found in the mid-rectum. Analyis by M-CE and in particular hrEUS allowed for appropriate resective strategy (Fig. 5.4).