Fig. 7.1
Aberrant arteriole in tumor. Aberrant small arteries in the lobules are useful features that support a diagnosis of neoplasm. However, this feature is not specific for tumors and should be used with other findings (see Fig. 7.2)
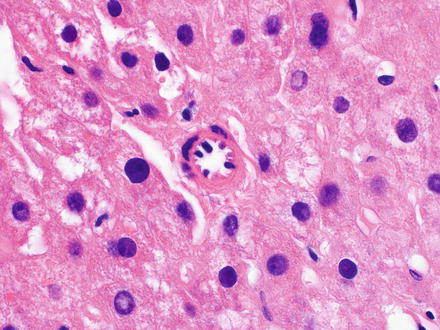
Fig. 7.2
Aberrant arteriole in non-tumor aberrant. Small arteries in the lobules can also be seen in non-neoplastic conditions, in this case in fatty liver disease
Cytological abnormities include increased nuclear-to-cytoplasmic ratios and nuclear atypia, but these features manifest differently in different tumors. For example, some hepatocellular carcinomas show little or no change in their nuclear-to-cytoplasmic ratio, while others show a clear reduction in tumor cytoplasm, often associated with increased cytoplasmic basophilia. Other cytological changes include eosinophilic inclusions, which can look like Mallory-Denk bodies (Fig. 7.3) or round hyaline inclusions (Fig. 7.4). Both hepatocellular carcinomas and fibrolamellar carcinomas can have pale bodies (Fig. 7.5). Some hepatocellular carcinomas may have clear cell change from glycogen, or may have fatty change. Nuclear atypia can be manifest by nuclear irregularities, variation in nuclear size, prominent nucleoli (Fig. 7.6), and occasionally as multinucleation (Fig. 7.7). Bile production is seen in a subset of cases (Fig. 7.8). Other cases may have striking lipofuscin deposition within the tumor cells.
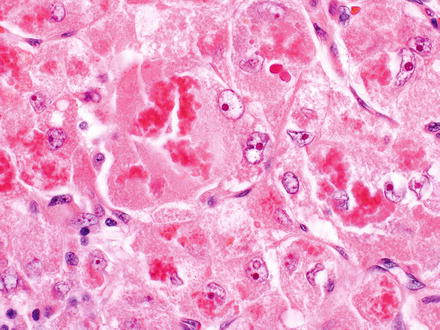
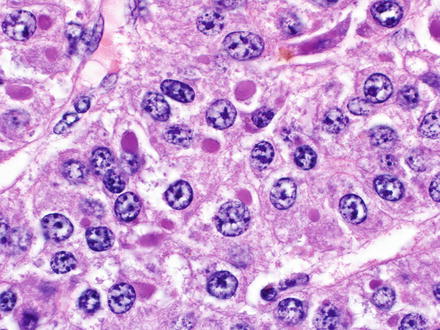
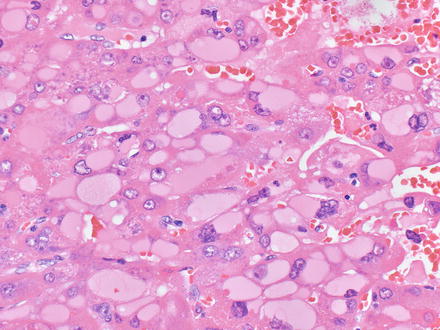
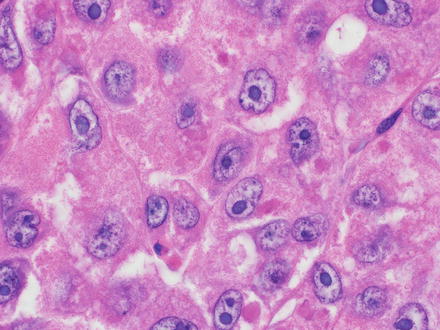
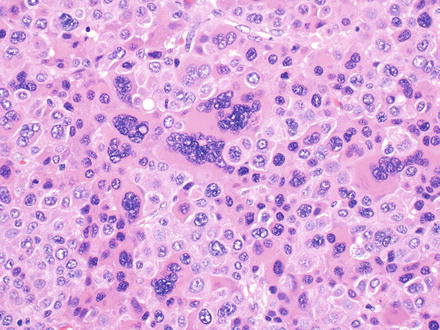
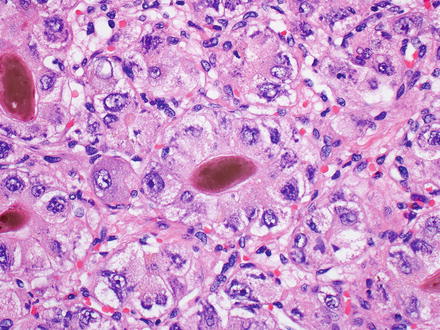

Fig. 7.3
Mallory-Denk bodies in hepatocellular carcinoma

Fig. 7.4
Eosinophilic cytoplasmic inclusions in hepatocellular carcinoma. Some studies suggest this finding has a worse prognosis

Fig. 7.5
Pale bodies in typical hepatocellular carcinoma. Pale bodies are neither sensitive nor specific for fibrolamellar carcinoma and are found in a subset of otherwise ordinary hepatocellular carcinomas

Fig. 7.6
Hepatocellular carcinoma, nuclear atypia. The nuclei show irregular membranes and prominent nucleoli

Fig. 7.7
Hepatocellular carcinoma, multinucleation. This hepatocellular carcinoma shows prominent multinucleation

Fig. 7.8
Hepatocellular carcinoma, bile production. Bile is seen in this hepatocellular carcinoma
7.4.2 Growth Patterns
Hepatocellular carcinomas have four major growth patterns: trabecular (Fig. 7.9), solid (Fig. 7.10), pseudoglandular (Fig. 7.11), and macrotrabecular (Fig. 7.12). Overall, the trabecular growth pattern is seen in approximately 70 % of cases, with about one-fifth of these having the macrotrabecular pattern. A solid growth is the dominant pattern in about 20 % of cases and the pseudoglandular pattern is dominant in 10 % [13]. Approximately 50 % of cases have mixed patterns in fully resected specimens. The solid growth pattern is also referred to as “compact” in some studies. Some studies also use the term “acinar” or “pseudocyst” when the pseudoglands are large in size, often filled with thin, granular material (Fig. 7.13). Macrotrabecular growth is variably defined, but typically has trabeculae that are at least 10 cells in thickness. The macrotrabecular pattern has a worse prognosis, at least when compared to the solid growth pattern [14]. Most cases with both the macrotrabecular pattern and basophilic tumor cells have elevated serum AFP levels and extensive angiolymphatic invasion. Other changes that are occasionally present in hepatocellular carcinoma include peliosis-like areas, with large pools of blood between tumor trabeculae (Fig. 7.14).
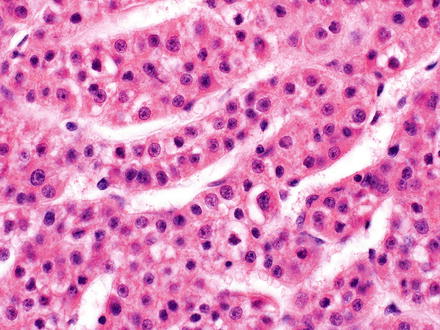
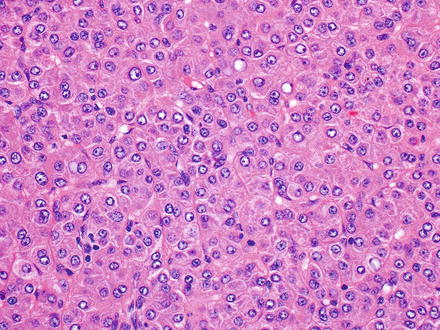
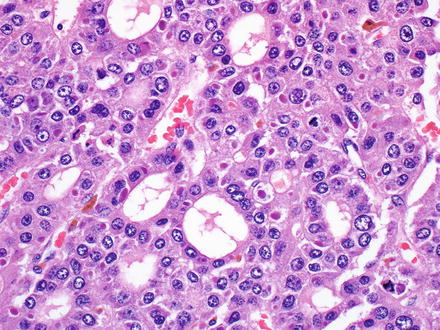
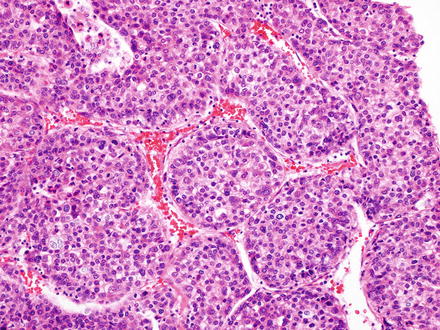
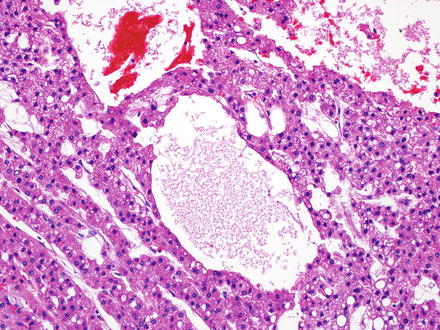
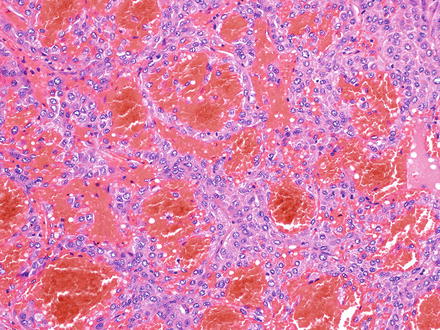

Fig. 7.9
Hepatocellular carcinoma, trabecular growth pattern

Fig. 7.10
Hepatocellular carcinoma, solid growth pattern

Fig. 7.11
Hepatocellular carcinoma, pseudoglandular growth pattern. Studies have shown that this pattern is statistically associated with beta-catenin mutations

Fig. 7.12
Hepatocellular carcinoma, macrotrabecular growth pattern. Some studies have shown that this pattern has a worse prognosis

Fig. 7.13
Hepatocellular carcinoma, pseudocyst. Pseudocysts are often filled clusters of red blood cells along with thin granular material, that likely represents altered proteins and red blood cells

Fig. 7.14
Hepatocellular carcinoma, peliotic-like areas
In addition to these four patterns, some hepatocellular carcinomas have nodules of distinctly different tumor morphology, often with higher-grade nuclear cytology, suggesting tumor evolution, with the emergence of a more aggressive clone. This finding is often called “nodule within a nodule” (Fig. 7.15) and can feature different combinations of all of the cytological changes and growth patterns discussed above. Interestingly, some patterns tend to co-occur more frequently, also supporting the supposition that this represents tumor evolution.
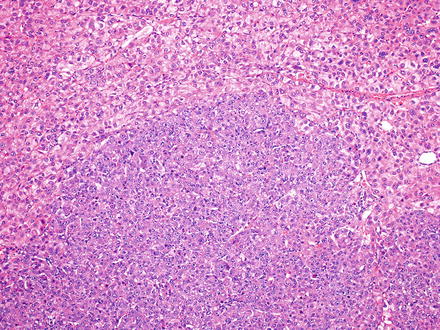

Fig. 7.15
Hepatocellular carcinoma, nodule within a nodule. Two distinct tumor morphologies are seen. While they share similarities, the different morphologies tend to have a sharp interface, with little or no transition
7.4.3 Changes After Chemoembolization Therapy
Many resection specimens will have been treated prior to surgery. The histological changes resulting from chemotherapy can include hemorrhage, necrosis, inflammation, and fibrosis. Embolic beads can also be seen (Figs. 7.16 and 7.17). It is possible that treatment could also change tumor morphology or tumor grade to some degree, but this has not been well studied. Treated tumors should be staged in the usual fashion, but the pathology report should also comment on tumor necrosis. At a minimum, the report should indicate whether there is total necrosis, some necrosis present with viable tumor, or only viable tumor with no necrosis. While the clinical significance of the percent necrosis is not clear, many clinicians appreciate more detailed information, for example, estimating the percent necrosis to the nearest 10 %. The necrosis can be estimated from the gross specimen or can be estimated by “averaging” the percent necrosis on the H&E stains. A gross examination is particularly helpful for estimating the percent necrosis, as sections often focus on the viable areas of the tumor. Of course, sample the tumor well. It is understood that the pathologist cannot distinguish treatment-related tumor necrosis from spontaneous tumor necrosis and all necrosis is counted when providing the percent tumor necrosis.
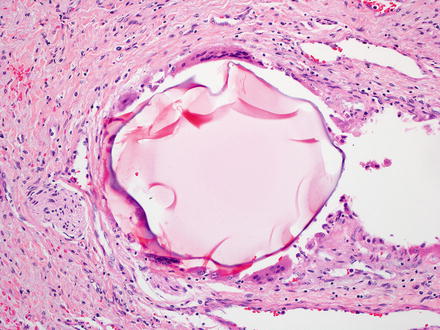
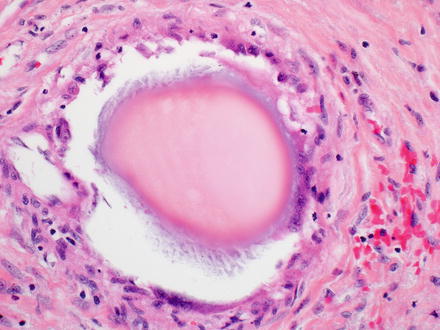

Fig. 7.16
Beads from chemoembolization. The embolization beads can have different histological appearances and often have a mild giant cell reaction at their edges

Fig. 7.17
Beads from chemoembolization. Another example of an embolization bead
7.4.4 Background Liver
In resection specimens, sections should be taken from the background liver to examine for active injury (fat, inflammation, cholestasis, granulomas, etc.) and for fibrosis. Many hepatocellular carcinomas have a rim of adjacent non-neoplastic liver tissue that can be inflamed and fibrotic because of proximity to the tumor, but this rim of tissue is often not representative of the background liver. Thus, sections to examine the background liver should be taken as far away from the tumor as possible, ideally at least 1 cm. Also, sections of the non-neoplastic liver that are taken from the cauterized margins can be important for margin evaluation, but are less useful for determining the fibrosis stage and underlying liver disease.
7.5 Histochemical Stains and Immunostains
7.5.1 Overview of Special Stains
Additional stains are useful tools for evaluating hepatic tumors. Currently, immunostains in surgical pathology are performed principally for diagnosis or subclassification of tumors, but in the future it seems likely that immunostains will also help select therapy as well as provide prognostic information.
In current practice, stains are used to address one of two questions. The first question is whether tissue that is clearly hepatocellular is benign or malignant. The second question is whether tissue that is clearly cancer is hepatocellular, biliary, or other (such as metastatic in origin). The stains performed on a given case will depend substantially on which question is being addressed. A common mistake is not making this distinction up front, which often leads to unnecessary stains, and sometimes to exhaustion of the block in biopsy material before a definite diagnosis can be made.
When the biopsy shows lesional tissue that is clearly hepatocellular in differentiation, then the differential in non-cirrhotic livers is focal nodular hyperplasia, hepatic adenoma, and hepatocellular carcinoma. When the lesion occurs in a cirrhotic liver, then the differential shifts to primarily that of a macroregenerative nodule, dysplastic nodule, and hepatocellular carcinoma. Stains that help with the differential diagnosis for cases that show obvious hepatocellular differentiation are shown in Table 7.1.
Table 7.1
Stains that can be helpful to distinguish benign from malignant liver lesions
Stains | Comment |
|---|---|
Reticulin | A small proportion of hepatocellular carcinomas has no obvious reticulin loss |
Ki-67 | Only helpful if significantly higher than background liver |
CD34 | Strong diffuse staining favors hepatocellular carcinoma, but on biopsy does not clearly distinguish adenoma from hepatocellular carcinoma |
Glypican-3 | Positive in about 80–90 % of hepatocellular carcinoma. Can be positive in benign livers with significant inflammation, in dysplastic nodules, and rarely in cirrhotic nodules |
Alpha-fetoprotein | Negative in benign lesions; positive in about one-third of all hepatocellular carcinomas |
When the biopsy shows tissue that is clearly carcinoma, but it’s not clear if the tumor is hepatocellular, then stains for hepatocellular differentiation are helpful (Table 7.2). The use of each stain is discussed further below. Each laboratory has its own somewhat unique experience with a given stain, based partly on antibodies, antigen retrieval, and other local methodology practices. Thus, the published literature for a given immunostain should be interpreted in association with direct experience.
Table 7.2
Stains to identify hepatocellular differentiation in an obvious malignancy
Stains | Comment |
|---|---|
HepPar1 | Positive in 90 % of all hepatocellular carcinomas; poorly differentiated hepatocellular carcinomas are most likely to be negative |
Arginase-1 | Positive in 90 % of all hepatocellular carcinomas; poorly differentiated hepatocellular carcinomas are most likely to be negative |
Glypican-3 | Positive in about 80–90 % of hepatocellular carcinomas. Can be positive in other types of cancer |
In situ hybridization for albumin | Limited availability also stains a subset of cholangiocarcinomas |
Polyclonal CEA (canalicular pattern) | Positive in 60–80 % of all hepatocellular carcinomas; poorly differentiated hepatocellular carcinomas are most likely to be negative |
CD10 (canalicular pattern) | Performance is similar to polyclonal CEA |
HBsAg | Can occasionally be helpful for poorly differentiated cancers in individuals with chronic hepatitis B |
Alpha-fetoprotein | Positive in only about one-third of all hepatocellular carcinomas |
A key point in using immunostains is to use them in conjunction with the H&E findings; the stains are much more powerful when used with H&E findings and, in turn, the H&E findings are strengthened when supported by immunohistochemistry. As one example, stains that detect hepatocellular differentiation, such as glypican-3, HepPar1, and arginase-1, can all be positive in carcinomas arising outside the liver. This diagnostic pitfall is important to know, but does not negate the value of these stains, as most of these carcinomas do not look very much like hepatocellular carcinoma on the H&E. That being said, there remain some very challenging cases, which have morphology that overlaps with both hepatocellular carcinoma and metastatic tumor from other sites. In some of these rare and difficult cases, the morphology and immunostains will be insufficient to make a definite diagnosis without incorporating clinical and radiological findings.
There is a large body of literature that compares the sensitivity and specificity of one stain versus another in diagnosing hepatocellular carcinoma. These studies are important and provide key insights into a stain’s performance, but there are five broad observations worth keeping in mind when considering these types of studies. First, despite any statistical superiority of one stain over another, this generally does not lead to the adoption of a single marker for diagnosis. Because of the complexities of tumor biology and the practical issues of diagnosis in surgical pathology, most expert liver pathologists prefer to use a panel of the top performing stains in their daily practice. Second, tumor grade influences a stain’s performance. One well-established example is that HepPar1 is more sensitive than glypican-3 in well-differentiated hepatocellular carcinomas, but glypican-3 is more sensitive than HepPar1 in poorly differentiated hepatocellular carcinomas. Third, other factors such as background fibrosis can also influence a stain’s performance. For instance, glypican-3 is more likely to be positive in hepatocellular carcinomas arising in cirrhotic livers than in those arising in non-cirrhotic livers [15]. It is not clear if these types of observations reflect differences in fundamental tumor biology, but they are relevant to interpreting the literature. Fourth, specific morphological patterns or variants of hepatocellular carcinoma may have their own staining characteristics. For example, HepPar1 is positive in only a subset of scirrhous hepatocellular carcinomas [16]. Fifth, it is broadly true of immunostains in general that the first reports in the literature will have the highest sensitivities and specificities. The sensitivities and specificities invariably drop off as additional studies are performed, with larger numbers of cases and experiences at various institutions. In fact, this rule is sufficiently strong that it characterizes essentially all known diagnostic immunostains in liver tumor pathology to date.
7.5.1.1 Cytokeratin Stains
Cytokeratin stains are typically used to prove epithelial differentiation in a poorly differentiated hepatocellular tumor, to examine the CK7/CK20 profile in cases thought to be potentially metastatic, or to test for CK19 expression in hepatocellular carcinoma (a marker of adverse prognosis).
Benign hepatocytes express CK8 and 18 and these keratins are also positive in almost all hepatocellular carcinomas. Likewise, CAM5.2, which targets primarily CK8 and to some degree CK7, is positive in essentially all hepatocellular carcinomas [17]. Hepatocellular carcinomas can be CK7 and/or CK20 positive; these stains are not useful to distinguish hepatocellular carcinoma from non-hepatocellular carcinoma. Overall, 20–40 % of hepatocellular carcinomas are CK7 positive [18, 19]. Cholestatic tumors and those that arise in the young (age less than 40) are more likely to be CK7 positive [20]. CK20 positivity is seen in approximately 5 % of hepatocellular carcinomas and is usually found in tumors that are also CK7 positive. CK20 staining without CK7 staining is rare, but still compatible with hepatocellular carcinoma, as long as the diagnosis is fully supported by morphology and other stains. About 10–15 % of hepatocellular carcinomas are CK19 positive; these have a worse prognosis [18, 19, 21]. Similar to CK20 expression, CK19 expression is typically found in hepatocellular carcinomas that are also CK7 positive [18, 19].
7.5.2 Stains to Separate Benign from Malignant Hepatocytes
7.5.2.1 Reticulin Stain
Loss of reticulin suggests hepatocellular carcinoma.
The reticulin stain has been used to diagnose hepatocellular carcinoma for nearly 40 years [22–24] and continues to be one of the most widely used diagnostic tools to distinguish well-differentiated hepatocellular carcinoma from benign liver lesions. A reticulin stain in the normal liver will demonstrate hepatic trabeculae composed of single or double layers of cells. The same pattern is found in hepatic adenomas, in focal nodular hyperplasia, and in regenerative nodules. Non-neoplastic trabeculae can be somewhat thicker in rapidly regenerating livers (three to four cells), but this is rarely a diagnostic dilemma, as the clinical situations are distinct (mass versus acute liver injury). In hepatocellular carcinoma with trabecular growth patterns, the hepatic trabeculae are thickened, which can be highlighted by the reticulin stain. Since many hepatocellular carcinomas do not have a trabecular growth pattern, the diagnostic construct of “reticulin loss” is also used to support a diagnosis of hepatocellular carcinoma. In benign livers and benign liver tumors, essentially all hepatocytes have reticulin on at least one of their borders (Fig. 7.18). In contrast, hepatocellular carcinomas show a loss or reduction in the amount of reticulin, leading to aggregates of hepatocytes that do not have any associated reticulin fibers (Fig. 7.19).
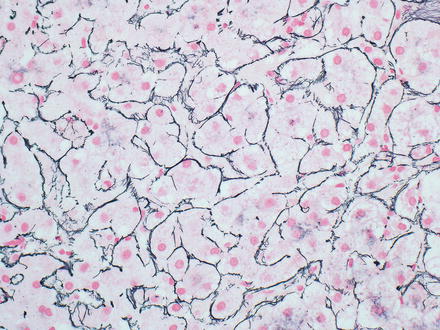
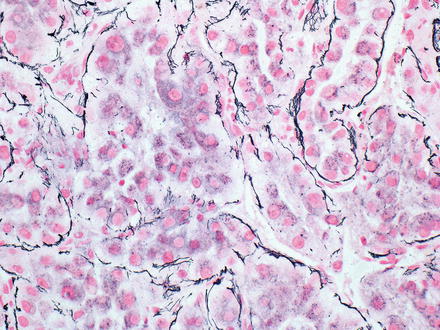

Fig. 7.18
Reticulin staining in non-neoplastic liver. There should be reticulin touching each hepatocyte

Fig. 7.19
Reticulin loss in hepatocellular carcinoma. Aggregates of hepatocytes have lost their association with reticulin. Same case as shown in Fig. 7.18. Reticulin stain interpretation benefits from having both non-tumor and tumor on same slide
There are several diagnostic pitfalls with the reticulin stain. First, a poor quality, or “light” reticulin stain can show artifactual reticulin reduction that mimics hepatocellular carcinoma. Therefore, it is important to carefully examine any non-neoplastic liver and/or control tissue on the slide for retention of normal reticulin staining. If the staining in the non-neoplastic liver and/or tissue control is diminished, the stain should be repeated. Second, reticulin loss should be more than focal and minimal to have diagnostic significance. Third, benign liver tissue with macrovesicular steatosis can show loss of reticulin, including both non-tumor tissue with fatty change (Fig. 7.20) and benign tumors with fatty change [24, 25]. In general, the reticulin loss in benign fatty tissue is more striking with higher amounts of steatosis [25]. Finally, a small proportion of well-differentiated hepatocellular carcinomas (less than 1 %) show no loss of reticulin staining on biopsy [26, 27]. In these cases, the diagnosis of hepatocellular carcinoma is made using other findings, including cytological atypia and increased proliferative rates.
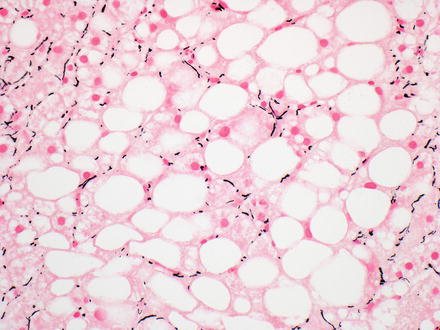

Fig. 7.20
Reticulin loss in non-tumor fatty liver. Reduction in the amount of reticulin is seen in this case of ordinary fatty liver disease, even though there is no tumor
7.5.2.2 CD34 Stain
Diffuse, strong sinusoidal staining suggests hepatocellular carcinoma.
CD34 highlights the sinusoids in zone 1 of the normal liver, but does not extend further into the lobules. In contrast, CD34 tends to show a strong diffuse sinusoidal staining pattern in hepatocellular carcinomas (Fig. 7.21) [28]. This strong and diffuse staining pattern is observed in nearly all moderately differentiated and most well-differentiated hepatocellular carcinomas. However, occasional hepatocellular carcinomas will lack this strong diffuse staining pattern [29], especially well-differentiated and poorly differentiated tumors. In addition, the macrotrabecular growth of hepatocellular carcinoma has a distinctive staining pattern, where CD34 highlights the very thick trabeculae (Fig. 7.22), but does not otherwise stain the tumor. Furthermore, metastatic carcinomas can occasionally have a diffuse sinusoidal staining patterns that mimic hepatocellular carcinoma. Finally, CD34 staining in both focal nodular hyperplasia and in hepatic adenomas is generally patchy, in contrast to the diffuse pattern of staining seen in hepatocellular carcinomas. However, occasional hepatic adenomas and focal nodular hyperplasias can have a diffuse pattern that is essentially indistinguishable from hepatocellular carcinoma [29–32]. For example, one study found diffuse sinusoidal CD34 staining in 27 % of hepatocellular adenomas, which correlated with beta catenin activated or unclassified type of adenomas [32]. For all of these reasons, CD34 is used less often than some of the other available stains and is never used alone, but can still be useful.
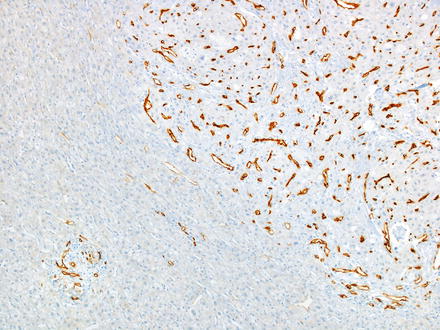
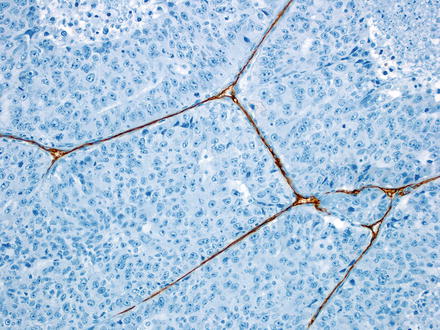

Fig. 7.21
CD34 staining in hepatocellular carcinoma. A strong diffuse sinusoidal pattern is seen within the tumor, while the non-neoplastic liver on the left of the image shows only zone 1 staining

Fig. 7.22
CD34 staining in macrotrabecular growth pattern. The large macrotrabecula are highlighted, but the typical diffuse sinusoidal staining pattern is not seen
7.5.2.3 Ki-67
Increased staining in tumor nuclei, relative to non-tumor tissue, suggests malignancy.
Immunohistochemical stains for Ki-67 can be helpful for distinguishing well-differentiated hepatocellular carcinoma from benign hepatocellular tumors, but a Ki-67 is most useful when combined with other stains. A high Ki-67 proliferative index favors hepatocellular carcinoma over adenoma, while a low proliferate rate can be seen in both adenomas and carcinomas. There is no well-established diagnostic cutoff, but in general, hepatic adenomas have a proliferative rate of less than 2 %. Hepatocellular carcinomas tend to have a biphasic distribution of proliferative rates, with about half of tumors falling between 20 and 60 % and half less than 10 % [33, 34]. A Ki-67 is most helpful when both tumor and non-tumor tissue are present on the same section.
7.5.2.4 Glypican-3
Cytoplasmic staining suggests cells are malignant. Glypican-3 is also used as evidence for hepatocellular differentiation (discussed in next section). Be aware that lipofuscin can also be glypican-3 positive.
Glypican-3 can occasionally be useful in distinguishing benign lesions from malignant lesions in non-cirrhotic livers. However, it is only informative when positive, as negative staining is seen in benign tumors as well as approximately 50 % of well-differentiated hepatocellular carcinomas. A positive immunostain result supports a malignant diagnosis because hepatic adenomas and focal nodular hyperplasia should be negative for glypican-3. This stain, however, is not helpful in distinguishing dysplastic nodules from hepatocellular carcinoma, as glypican-3 is positive in about 50 % of high-grade dysplastic nodules and 5 % of macroregenerative/low-grade dysplastic nodules [15]. Glypican-3 can also stain benign hepatocytes in markedly inflamed livers [35].
7.5.3 Stains for Hepatocellular Differentiation
The most commonly used immunostains to identify hepatocellular differentiation are CD10, polyclonal CEA (pCEA), HepPar1, glypican-3, and arginase-1. Alpha-fetoprotein is generally not used because of low sensitivity, but occasionally can help. These stains are powerful tools to identify hepatocellular differentiation. Nonetheless, no single stain is a panacea for all difficult cases; such cases are often best approached using a panel of stains, in conjunction with the H&E morphology and, when available, the clinical findings and imaging tests. A commonly used panel would be HepPar1, arginase-1, and glypican-3.
7.5.3.1 pCEA and CD10
Canalicular staining supports hepatocellular differentiation. Membranous and cytoplasmic staining is compatible with, but not specific for, hepatocellular differentiation.
Immunostains for both CD10 and pCEA identify hepatocellular differentiation by a distinctive canalicular staining pattern (Fig. 7.23). The overall sensitivity of CD10 and pCEA canalicular staining for hepatocellular carcinoma is about 55 % and 75 %, respectively. An additional 10–15 % of cases have either membranous or cytoplasmic staining. These latter patterns are compatible with hepatocellular carcinoma, but are not specific, as cytoplasmic and membranous staining can also be seen with other carcinomas. The membranous and cytoplasmic staining patterns are often referred to as “non-canalicular” in the literature. They often coexist with canalicular staining patterns (about a third of all cases), but a combination of canalicular and non-canalicular staining still provides specific support for a diagnosis of hepatocellular carcinoma.
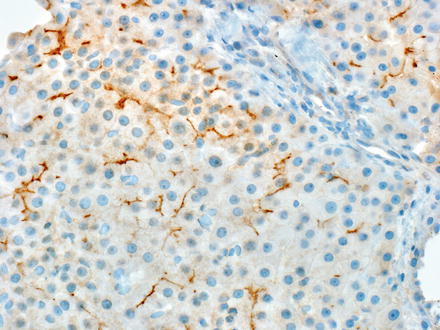

Fig. 7.23
CD10 staining in hepatocellular carcinoma. A distinctive branching canalicular pattern of staining is needed for evidence of hepatic differentiation
Both stains perform best on well-differentiated and moderately differentiated hepatocellular carcinomas, but there is a significant drop in sensitivity for poorly differentiated hepatocellular carcinomas. For this reason, lack of canalicular staining with pCEA or CD10 immunostains provides stronger evidence against a diagnosis of hepatocellular carcinoma in a well or moderately differentiated tumor than it does in a poorly differentiated tumor. In addition, canalicular staining patterns are often focal and equivocal in poorly differentiated tumors and can be hard to confidently identify, with significant inter- and intraobserver variability.
7.5.3.2 HepPar1
Granular, cytoplasmic staining indicates hepatocellular differentiation.
The HepPar1 immunostain revolutionized surgical pathology by providing a highly specific stain for hepatocellular differentiation [36]. HepPar1 recognizes a mitochondrial antigen in the same metabolic pathway as arginase-1 [37]. The staining is cytoplasmic and granular, with 85–95 % of hepatocellular carcinomas staining positive [38]. As is true for most stains that identify hepatocellular differentiation, HepPar1 performs best in well and moderately differentiated tumors, but has a lower of sensitivity for poorly differentiated tumors. Staining is typically easy to interpret as either positive or negative, giving it an advantage over pCEA and CD10, where identification of the canalicular pattern can sometimes be challenging.
Of note, HepPar1 staining is patchy in some moderately differentiated and poorly differentiated hepatocellular carcinomas, but diffuse staining is not necessary for a diagnosis. An important diagnostic pitfall, however, is that benign hepatocytes can be entrapped within a tumor and these hepatocytes will be strongly HepPar1 or arginase-1 positive (Figs. 7.24 and 7.25). Thus, it is always important to revisit the H&E stain and make sure that any patchy HepPar1 staining is not simply entrapped hepatocytes. HepPar1 is still very useful in needle biopsies and fine needle aspirates, as studies of HepPar1 with this material generally report broadly similar results to those of studies based on resections. However, if tumor tissue is very scant, then sensitivity could be adversely affected.
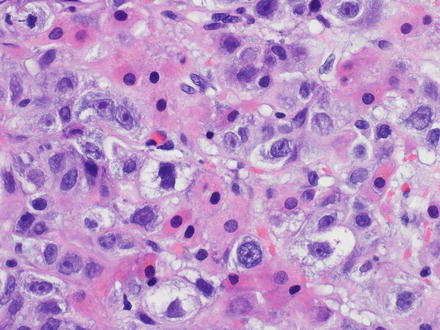
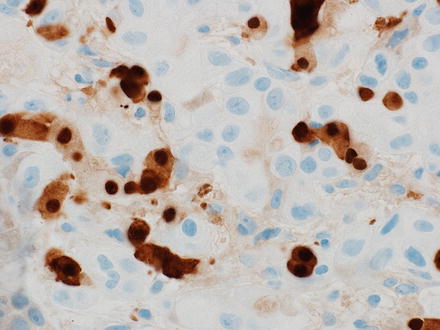

Fig. 7.24
Entrapped hepatocytes mimicking hepatocellular carcinoma. This poorly differentiated metastatic prostate carcinoma had entrapped hepatocytes, which are seen as the smaller cells with eosinophilic cytoplasm in this image

Fig. 7.25
Entrapped hepatocytes in metastatic carcinoma. The benign hepatocytes are Arginase positive, same case as Fig. 7.24
There are a wide variety of other carcinomas that can be HepPar1 positive [39], but the most common are gastric (up to 40 %), ovarian clear cell and ovarian mucinous (up to 35 % each), adrenal cortical carcinoma (up to 15 %), lung adenocarcinoma (up to 15 %), cholangiocarcinoma (up to 10 %), and neuroendocrine carcinoma (up to 10 %). The good news is that by and large these tumors typically do not have H&E findings that resemble hepatocellular carcinoma and HepPar1 performs very well in cases in which the H&E differential includes hepatocellular carcinoma. Nevertheless, some of these tumors can mimic hepatocellular carcinoma, in particular oncocytic tumors such as neuroendocrine carcinomas and adrenal cortical carcinomas. Thus, on challenging cases a panel of immunostains is typically the best approach.
7.5.3.3 Glypican-3
Cytoplasmic staining supports hepatocellular differentiation. Be aware that lipofuscin is also glypican-3 positive.
Glypican-3 shows cytoplasmic granular staining and is positive in approximately 80–90 % of hepatocellular carcinomas. Glypican-3 staining is often patchy and can be patchier than HepPar1. Patchy staining can be an issue with needle biopsies and fine needle aspirates, with some studies showing positivity in only 50 % of cases [40].
Glypican-3 does not normally stain the background liver, but the background liver can be focally glypican-3 positive when there is significant inflammation [35]. Hepatic adenomas and focal nodular hyperplasias are always negative for glypican-3 [29, 41, 42]. However, glypican-3 can stain lipofuscin (Fig. 7.26). Many adenomas and focal nodular hyperplasias have abundant lipofuscin and care must be taken to make sure that any glypican-3 staining is not simply lipofuscin. In cirrhotic livers, macroregenerative nodules and dysplastic nodules can be glypican-3 positive [41], making the stain less helpful in separating benign from malignant nodules in this setting.
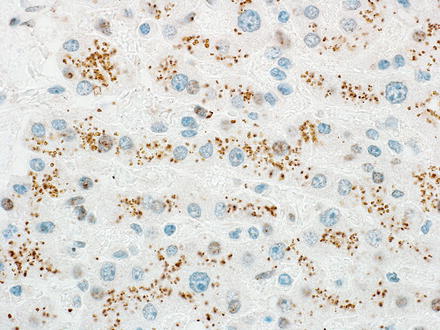

Fig. 7.26
Glypican-3 and lipofuscin. The lipofuscin in this hepatic adenoma is positive for glypican-3, an important diagnostic pitfall
In contrast to all of the other commonly used markers for hepatocellular differentiation, glypican-3 performs poorly in very well-differentiated hepatocellular carcinomas, with only 50 % positivity [41]. Other factors that reduce the frequency of glypican-3 staining in hepatocellular carcinoma include non-cirrhotic background liver [41] and scirrhous morphology [16]. On the other hand, glypican-3 positivity is more likely in hepatitis B-related hepatocellular carcinoma [43].
Even a cursory review of the literature reveals that many tumors from most organ systems have at least one study showing that a proportion of cases are glypican-3 positive. In particular, these include yolk sac tumors (95–100 %), squamous cell carcinoma of the lung (approximately 50 %), chromophobe renal carcinoma (about 40 %), and cholangiocarcinoma (about 10 %). As discussed with HepPar1 staining, this broad frequency of positivity in so many different malignancies is generally a manageable problem, as long as the H&E findings are fully taken into account and glypican-3 is used as part of a panel of stains.
7.5.3.4 Arginase-1
Staining can be cytoplasmic and/or nuclear and supports hepatocellular differentiation (Fig. 7.27).
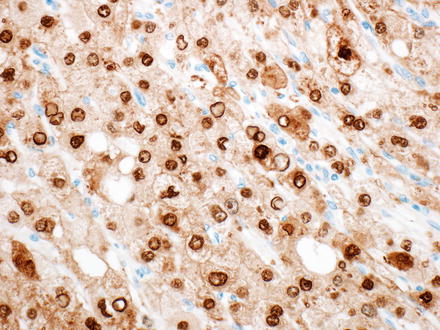

Fig. 7.27
Arginase. Arginase staining in hepatic tumors can be cytoplasmic or nuclear
Arginase-1 is a more recent addition to the diagnostic toolkit used to identify hepatocellular differentiation [44]. Arginase-1 shares broad similarities with HepPar1: both are positive in benign and malignant hepatocytes, both can be patchy in hepatocellular carcinoma, both have some drop in sensitivity with poorly differentiated hepatocellular carcinomas, and neither is absolutely sensitive or specific for hepatocellular carcinoma. To date, studies suggest arginase-1 has better performance characteristics than either glypican-3 or HepPar1 in poorly differentiated hepatocellular carcinomas. However, a small proportion of hepatocellular carcinomas are negative for arginase-1 but positive for HepPar1 or glypican-3. For well or moderately differentiated tumors, where the H&E findings strongly suggest hepatocellular carcinoma, a single (HepPar1 or arginase-1) immunostain can be sufficient evidence to confirm hepatocellular lineage. Immunostains are helpful even in well-differentiated carcinomas, as some metastatic tumors can closely mimic well-differentiated hepatocellular carcinoma, in particular a subset of neuroendocrine tumors and oncocytic renal cell carcinomas (Fig. 7.28).
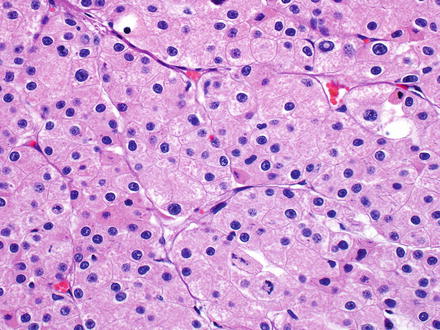

Fig. 7.28
Metastatic chromophobe carcinoma, mimicking hepatocellular carcinoma. This tumor closely mimics a well-differentiated hepatocellular carcinoma. Negative HepPar1 staining helped lead to the correct diagnosis
7.5.3.5 Albumin In Situ Hybridization
Staining is cytoplasmic and supports hepatocellular differentiation.
In situ hybridization for albumin is another technique to demonstrate hepatocellular differentiation. This assay is also positive in many cholangiocarcinomas, albeit often at a lower level of expression than hepatocellular carcinomas. Depending on the methodology, a small subset of neuroendocrine tumors can also be positive.
7.5.3.6 Alpha-fetoprotein
Cytoplasmic staining can support a diagnosis of hepatocellular carcinoma.
Alpha-fetoprotein is positive in approximately one-third of hepatocellular carcinomas and can be useful when positive, but the large number of negative cases makes it a less useful immunostain. Nonetheless, in occasional cases that are very poorly differentiated, an immunostain for alpha-fetoprotein can still help support the diagnosis. Immunostains for alpha-fetoprotein often have high background, and the staining should be strong and bright and clearly more intense than the background to be called positive.
7.5.3.7 Other Immunostains
In working up a poorly differentiated tumor, it is not uncommon to use a variety of immunostains and then wonder about their staining characteristics in hepatocellular carcinomas. A table of commonly encountered situations is provided (Table 7.3).
Table 7.3
Frequency of positive staining for non-hepatocellular markers in hepatocellular carcinoma
Immunostain | Approximate frequency (%) | Comments |
|---|---|---|
CDX2 | 5 | Staining is often focal in hepatocellular carcinoma. Also, about 30 % of cholangiocarcinomas are CDX2 positive |
CD117 (c-Kit) | 70 | |
CD138 | 65 | Non-neoplastic hepatocytes are also positive. Membranous staining is seen in both tumor and non-tumor |
CAM5.2 | >99 | |
Cytokeratin AE1/AE3 | 10 | |
CK19 | 15 | Positive tumors have a worse prognosis. Positive cases typically co-express CK7 |
CK7 | 30 | Hepatocellular carcinomas that are cholestatic or occur in younger individuals (<40) are positive in the majority of cases |
CK20 | 10 | Positive cases typically co-express CK7 |
EMA | 5 | Positive in most renal cell carcinomas, so can be helpful when evaluating a clear cell hepatocellular carcinoma |
Glutamine synthetase | 50 | |
Moc31 (anti-EpCam) | 35 | |
SALL4 | 45 | May be a marker of “stemness” |
Villin | 30 | Staining is typically strong and cytoplasmic and can have a membranous accentuation |
Vimentin | 10 | About 10 % of typical hepatocellular carcinomas are positive. Sarcomatoid hepatocellular carcinomas are positive in most cases. Vimentin expression is associated with more aggressive behavior |
WT1 | >80 |
7.6 Differential Diagnosis
The differential diagnosis for a hepatocellular neoplasm depends on the presence or absence of cirrhosis in the background liver and the degree of tumor differentiation. For well-differentiated hepatocellular tumors in non-cirrhotic livers, the differential is primarily focal nodular hyperplasia, hepatocellular adenoma, and well-differentiated hepatocellular carcinoma. Hepatic adenomas and focal nodular hyperplasia should have no or minimal cytological atypia, inconspicuous nucleoli, no mitotic activity and a low (typically less than 2 %) Ki-67 proliferative rate. As a caveat, areas next to tumor necrosis in adenomas may show slightly higher proliferative rates. Other clues include that hepatocellular adenomas are rarely cholestatic and rarely if ever have a pseudoacinar growth pattern, unless they are androgen related.
Neither adenomas nor focal nodular hyperplasia have cytoplasmic inclusions and neither have reticulin loss. Reticulin loss needs to be definite to support a diagnosis of hepatocellular carcinoma. The significance of very focal or otherwise equivocal reticulin loss is less clear and by itself does not warrant a diagnosis of carcinoma. Also of note, the reticulin framework can be disrupted near areas of hemorrhage and necrosis. As another caveat, both adenomas and focal nodular hyperplasias can have fatty change, and the areas of fatty change may have foci of reticulin reduction that is due to the steatosis and not an indicator of carcinoma. However, extensive or diffuse loss of reticulin should not be seen in benign tumors, even when fatty.
Clinical information can be very valuable when evaluating well-differentiated tumors. In fact, if the clinical background is unknown, or if the clinical setting is atypical for hepatocellular adenoma, then it is worth approaching the case with extra care. Rare tumors are simply not classifiable on biopsy despite a full histological evaluation and in these cases one approach is to use the term “well-differentiated hepatocellular neoplasm” and give a prioritized differential in a note. In fact, even after resection and complete histological evaluation, there is a small subset of well-differentiated hepatocellular neoplasms that have a mixture of features that put them on the border between adenoma and well-differentiated hepatocellular carcinoma. These tumors arise in livers with no or minimal fibrosis, typically have definite but very mild cytological and/or architectural atypia, and have focal or rare small foci of reticulin reduction. Immunostains show a low proliferative rate by Ki-67, negative glypican-3, and negative alpha-fetoprotein. Many of these tumors are associated with some of the classic risk factors for hepatic adenomas and can be designated as atypical hepatocellular adenomas or hepatocellular tumors of uncertain malignant potential (HUMP) [45]. However, this term should be used only after a complete histological evaluation of the tumor specimen. Consultation with colleagues with expertise in liver pathology can also be helpful in these cases.
In cirrhotic livers, the differential diagnosis for a well-differentiated hepatocellular tumor is primarily between a macro-regenerative nodule, dysplastic nodule, and well-differentiated hepatocellular carcinoma. Finding portal tracts within the lesion favors a benign nodule, while reticulin loss supports hepatocellular carcinoma. A proliferative rate that is significantly higher in the tumor than the background liver also supports a diagnosis of hepatocellular carcinoma. Positive staining for glypican-3 is not as helpful in this setting if used in isolation, as glypican-3 can be positive in some dysplastic nodules and negative in many well-differentiated hepatocellular carcinomas. A CK7 immunostain can also be helpful, as regenerative and dysplastic nodules retain a rim of reactive bile ducts in most cases, while many hepatocellular carcinomas do not.
7.7 Prognosis
The median survival following a diagnosis of hepatocellular carcinoma is less than a year in most studies. At the time of presentation, the single most important prognostic factor is whether the tumor can be resected. Resection with curative intent has a 5 year survival that ranges from 25 to 50 %, with a survival rate that approaches 80 % for small tumors measuring less than 2 cm [46]. However, most individuals are not candidates for resection at the time of presentation. Liver transplantation for hepatocellular carcinoma has a 5-year survival of approximately 75 %, similar to those transplanted without hepatocellular carcinoma [46], and has the added benefit of curing the underlying cirrhosis, but this option is available to few individuals because of organ shortage and other medical conditions. Overall, transarterial chemoembolization (TACE) is the most common treatment for hepatocellular carcinoma, followed by various methods of ablation.
After surgery, tumor recurrence is most commonly seen in the liver. Based on clonality studies, tumor recurrence is clonally related to the original tumor in about two-thirds of cases, while the remaining third of cases represents development of a second primary [47, 48].
Good prognostic factors include clinical parameters such as young age, female gender, and lack of other significant comorbidities. Pathology findings also strongly influence survival. In the background liver, advanced fibrosis or cirrhosis is an adverse prognostic marker [49]. The three most significant prognostic factors in tumor pathology are tumor size, tumor grade, and angiolymphatic invasion. These three key factors are all correlated, with larger tumors more likely to be poorly differentiated and more likely to have angiolymphatic invasion. However, each has independent prognostic value and each is scored separately. Tumor subtype also has prognostic relevance.
7.7.1 Size
Tumor size is one of the most important prognostic factors and is incorporated into all staging systems, with larger tumors having worse prognosis. Tumor size is defined as the maximum diameter measured in centimeter. In many studies there appears to be a threshold effect of about 4 cm, with tumors from 1 to 4 cm having no incremental decrease in survival for each additional centimeter. In contrast, tumors greater than 5 cm have an incremental decrease in survival for each centimeter of increased size. Multifocality is also a poor prognostic indicator.
7.7.2 Angiolymphatic Invasion
Vascular invasion is powerful predictor of prognosis after both resection [50, 51] and liver transplantation [52]. The one exception is for tumors less than 2 cm, where the prognosis remains favorable even with microvascular invasion [53].
Macrovascular invasion is defined as tumor involving vessels that is recognized by gross examination or by imaging. The frequency of macrovascular invasion ranges considerably, but is between 5 and 30 % in most studies. Microvascular invasion can only be identified histologically and is seen in approximately 30 % of resected specimens, though the frequency ranges in studies from 15 to 57 % [54]. Microvascular invasion is best identified in sections taken at the tumor–non-tumor interface and this area of the specimen should be well sampled. Vascular invasion most commonly involves the portal veins and central veins and less commonly the hepatic arteries. Arterial invasion is generally considered to carry a worse prognosis than venous invasion.
There is potential for additional prognostic information in several other features of vascular invasion, though they are not routinely captured in surgical pathology reports. First, the number of vessels with invasion is prognostically relevant, with more vessels involved having a worse prognosis. However, a well-defined cutoff to separate focal vascular invasion from more extensive invasion has not yet been developed and validated. In addition, some studies have suggested that the size of tumor deposits in vessels is prognostic, with deposits of greater than 50 cells having a worse prognosis (Fig. 7.29) [55]. Furthermore, prognosis is worse when there is angiolymphatic invasion of vessels greater than 1 cm away from the primary tumor or when invaded vessels have muscular walls [56].
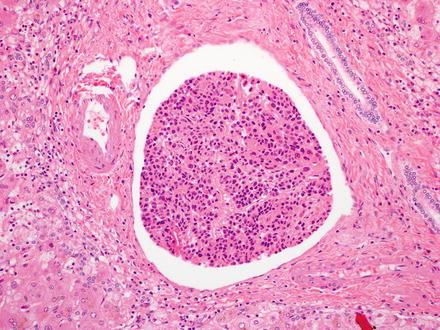

Fig. 7.29
Angiolymphatic invasion. Large groups of tumor cells carry a worse prognosis
Retraction artifact can closely mimic lymphatic or venous invasion, so an endothelial lining should be present to make the diagnosis. In addition, finding rare individual or small clusters of tumor cells floating freely in an otherwise empty vascular lumen is generally interpreted as sectioning artifact and not true vascular invasion.
7.7.3 Tumor Grading
Tumor grade carries important prognostic value. Tumor grade predicts overall patient survival and disease free survival after resection for hepatocellular carcinoma in cirrhotic [50, 57] and non-cirrhotic livers [51], and after liver transplantation [50, 52]. Tumor grade correlates with tumor size, age at presentation, and angiolymphatic invasion, but has independent predictive value. The most widely used grading system in research studies is the Edmondson–Steiner grading system or the modified Edmondson Steiner grading system (Table 7.4) [13]. When two or more nuclear grades are present in a tumor, the tumor is classified according to the worse nuclear grade [58].
Table 7.4




Modified Edmondson–Steiner grading system for hepatocellular carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








