| 20 | Hemostasis |
The efficacy of endoscopic intervention in treating upper gastrointestinal bleeding and its benefits from a patient perspective have already been shown. Recently, these have also increasingly been demonstrated in lower gastrointestinal bleeding (15). There are basically three methods that are suitable for achieving hemostasis in the lower gastrointestinal tract: thermocoagulation (with and without tissue contact), injection therapy of various agents, and mechanical methods. Regardless which method is used, it is important that before endoscopic intervention, optimal visibility conditions are ensured and, in particular, that the suspected bleeding source is washed off. Some biopolar coagulation probes include an opening at the tip for irrigation as well as the necessary generator have pumps. However, there are also devices (Fig. 20.1) that can be attached at the opening of the instrument channel enabling forced irrigation. At the same time, probes can still be introduced into the instrument channel. In situations where vision is obscured, e.g., by stool, blood clots, and heavy bleeding, washing away the stool can be of great help.
Thermocoagulation
Thermal devices deliver heat directly (heater probe) or indirectly by tissue absorption of light energy (laser) as well as passage of electrical current through tissue (bipolar probe, argon plasma coagulation). Heat application causes edema, coagulation of tissue protein, and contraction of vessels in the tissue and thus hemostasis. Coagulation of tissue occurs at a temperature of at least 70 °C.
 Monopolar and Biopolar Electrocoagulation
Monopolar and Biopolar Electrocoagulation
In biopolar coagulation an electrical current passes through the tissue between the two electrodes contained in the probe tip (Fig. 20.2). This requires that the tissue not be desiccated (dried out) as this results in loss of conductivity. Unlike monopolar probe use, the current does not pass through the patient’s body, but is limited locally to the targeted tissue area. Loss of conductivity as tissue desiccation increases limits depth and breadth of tissue damage as well as maximum temperature (100 °C). Bipolar probes generally have an opening at the tip for irrigation (Fig. 20.2b), the effect of which is two-fold: improved visualization of the bleeding source and formation of a thin film of fluid separating the probe from the tissue and preventing adhesion. If the probe tip sticks to the tissue, the electrical current is interrupted and the probe must be removed from the endoscope and cleaned. Removal of the adhered probe in turn entails the additional risk of tearing tissue and inducing bleeding. One study reports this occurring in 18% of applications for peptic ulcers (19). Probes are also available which include an extendable needle at the tip (Fig. 20.3), allowing quick alternation between injection and coagulation therapy, which can have distinct advantages in critical situations.

Fig. 20.1 Endoscopic irrigation device (Endowasher, MTW). a Irrigation from the working channel of the colonoscope.
b Adaptor attachment placed on the working channel to transport irrigation water from the pump into the endoscope.
c Pump.
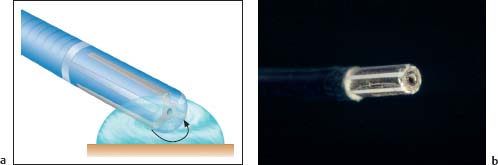
Fig. 20.2 Bipolar coagulation.
a Principle of bipolar coagulation. The electrical current flows (arrow) from the electrode in the probe tip through the tissue to the other electrode.
b ACMI bipolar probe. Steel electrodes lying lengthwise and parallel to one another in the tip.

Fig. 20.3 Injection Gold Probe (Boston Scientific).
a Probe with electrodes spiraling around the tip. On the left, the built-in needle is extended, allowing, e.g., epinephrine injection to achieve hemostasis and improve visualization. After injection, the needle is withdrawn and coagulation begins.
b Endoscopic view. Injection Gold Probe is placed on the mucosa (images courtesy of Boston Scientific).
Monopolar electrocoagulation. Monopolar electrocoagulation requires the placement of an indifferent plate (neutral electrode) on the patient’s body. The electrical current flows from the probe tip through the patient’s body. Some models of monopolar probes also have holes in the probe tip for irrigation, e.g., electrohydrothermal (EHT) probes. In principle, monopolar probes can be attached to any high frequency electrosurgery unit, as in papillotomy and polypectomy. Coagulation depth is greater in monopolar coagulation than in bipolar coagulation (31), though the level of energy application is more predictable in monopolar probes with irrigation at the tip (EHT probes) and it is also less dependent on angle of delivery (30). The problem with monopolar probes is that the electrical current flowing to the neutral electrode “flows away” and thus current density is much higher in the surrounding area compared with the coagulation site. In general, bipolar coagulation appears more appropriate for hemostasis in the colon as perforation risk—at least theoretically—appears lower.
Rebleeding after polypectomy. Postpolypectomy rebleeding can be managed by attempting to ensnare and coagulate the remaining portion of the stalk. This of course requires an adequately long polyp stalk. However, it is a simple method, which does not require extensive preparation and it can be effective in achieving hemostasis even in heavier rebleeding.
 Argon Plasma Coagulation (APC)
Argon Plasma Coagulation (APC)
Argon plasma coagulation (APC) transmits energy from ionized argon gas to the tissue without contact between the probe and tissue. Equipment includes a unit for controlling and regulating the supply of argon gas, a high frequency electrosurgical generator, and a flexible application probe (Fig. 20.4). The probe is a 2-mm-thick Teflon tube (Fig. 20.5), which is inserted into the endoscope’s working channel. Energy delivery from the probe tip can “go around a corner” and there are also probes that emit gas sideways, offering the potential of coagulation parallel to the probe axis.
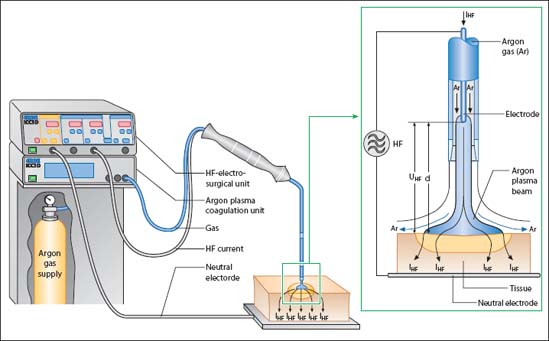
Fig. 20.4 APC unit (Erbe) comprising high frequency (HF) electrosurgical unit, argon plasma coagulation unit, argon gas tank, and probe. The lumen of the Teflon probe contains an electrode surrounded by argon gas. The distal end of the probe has a ceramic tip containing a tungsten electrode. The argon gas is ionized by the high electrical current passing the probe tip, and acts as a conductor. The ceramic tip prevents sticking on inadvertent tissue contact. The high frequency current is applied to the tissue surface via the ionized argon gas.
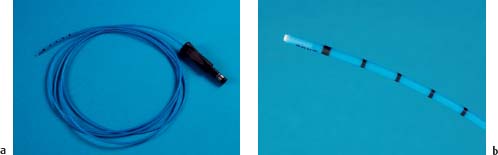
Fig. 20.5 APC probe (Erbe).
a Image showing the entire probe device.
b Close-up view of the probe tip.

Fig. 20.6 Device for suctioning vapor produced during APC or laser application.
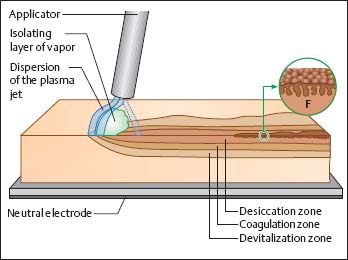
Fig. 20.7 Depth of devitalization and coagulation zones, schematic illustration. The evaporating liquid forms a cloud of vapor. Longer-lasting application causes formation of a spongelike dry structure (F) on the surface of parenchymatous organs. The electrically isolating vapor cloud and the formation of a desiccation zone together cause the plasma beam to automatically move over the entire reachable surface (based on 10).
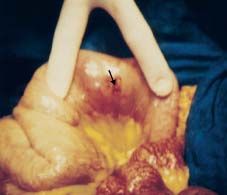
Fig. 20.8 Perforation from APC application in duodenum. The perforation site is marked with an arrow.
Complications and potential applications. Even without perforation, argon gas can permeate the colon wall. Occurrence of pneumoperitoneum and pneumomediastinum has been reported. Clinical way of acting is problematic and in such situations excluding perforation is nearly—if not entirely—impossible.
Argon plasma coagulation techniques can be applied in the colon for achieving hemostasis in bleeding angiodysplasias ( 20.1), diverticular hemorrhage, postpolypectomy rebleeding (Fig. 20.9), and tumor bleeding.
20.1), diverticular hemorrhage, postpolypectomy rebleeding (Fig. 20.9), and tumor bleeding.
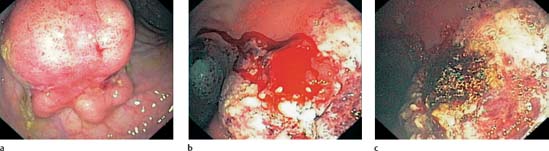
Fig. 20.9 APC application (Erbe) in rebleeding after polypectomy.
a Shortstalked rectal polyp.
b Hemorrhage from the polypectomy wound.
c Coagulation at the resection site achieves hemostasis.
 20.1 Coagulation of an angiodysplasia using APC (ERBE)
20.1 Coagulation of an angiodysplasia using APC (ERBE)
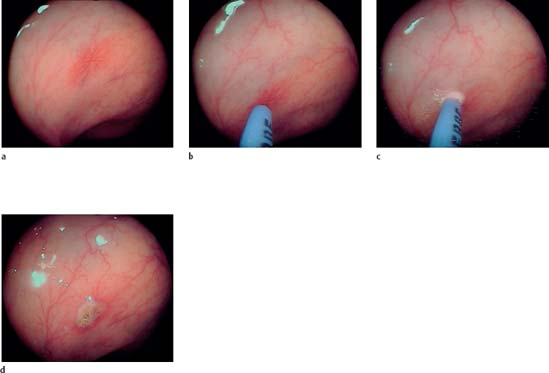
a–d Coagulation of an angiodysplasia (a) in the ascending colon. The probe is extended from the endoscope’s working channel to 2–10mm from the tissue (b). Energy application: tissue blanching is a sign of successful coagulation (c). After coagulation the angiodysplasia is no longer visible (d).
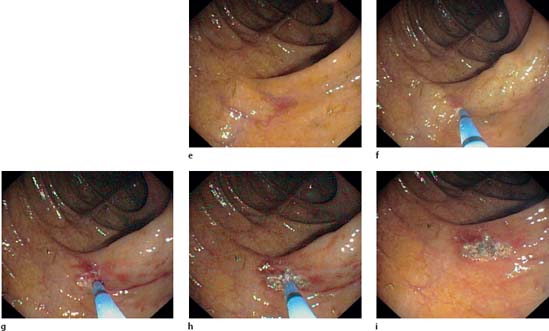
e–i Coagulation of an angiodysplasia (e) in the ascending colon. Energy application. Starting at the periphery, the vascular malformation is destroyed using brief pulses to effect coagulation (f–h). The bluish beam is easily visible at the probe tip (h). After coagulation the angiodysplasia is no longer visible (i).
 Laser
Laser
Laser is the acronym for “Light Amplification by Stimulated Emission of Radiation.” A crystal (active medium) is excited by outside energy to emit particularly intense light waves. One side of the crystal is completely reflective while the other is only partially reflective. The reflectivity of the crystal causes a type of chain reaction amplifying the light beam (optical pumping). Only the photons traveling perpendicular to the mirrors are amplified; all others escape to the side. When the beam has reached a specific intensity, it “shoots” through the partially mirrored frontal surface to the outside, creating a nearly parallel band of light which is comprised of only a single color (“monochromatic”) and in which the individual light waves vibrate in the same way (“coherent”). The intensity of the beam of light produced is much higher than in normal mixed-color light (Fig. 20.10). A flexible optical fiber transmits the laser beam.
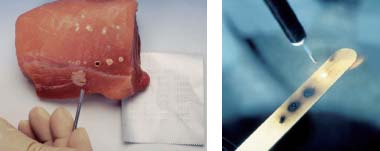
Fig. 20.10 Demonstration of the effect of a laser beam.
a Laser application on a piece of meat. Creation of exact coagulation points.
b Laser application on a wooden stick. Though the beam is invisible, it burns the wood.
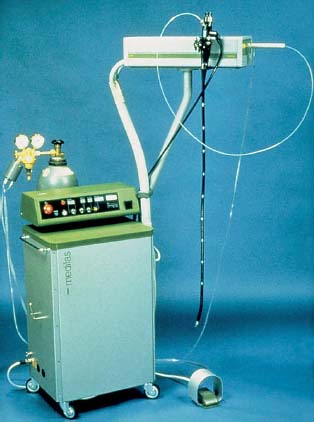
Fig. 20.11 Nd:YAG generator (MediLas, MBB).
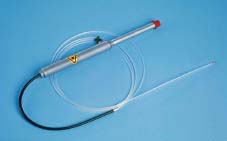
Fig. 20.12 Nd:YAG laser optical fiber (diameter: 2.1 mm) (GE 300 B, MBB). The optical fiber is inserted into the working channel of the endoscope.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






