Conventional diffusion-based dialysis modalities, including high-flux hemodialysis, are limited in their capacity to effectively remove larger-molecular-weight uremic toxins. Hemodiafiltration (HDF) is a modality that enhances convective solute transport of a broad range of uremic toxins known to be involved in uremia (Vanholder, 2003) and may provide patients with a number of benefits, including improved outcomes.
I. DIFFUSION VERSUS CONVECTION-BASED CLEARANCES. Hemodialysis relies on diffusive transport. The rate of diffusion of the molecules is inversely proportional to the square root of their molecular weight. Larger molecules have a relatively low speed of diffusion and therefore a relatively slow clearance by hemodialysis. Convective transport depends on solvent drag, where molecules, regardless of their molecular weight, are transported across the membrane by bulk fluid flow. The extent to which the solute is taken across the membrane depends on the so-called sieving coefficient, which varies from 0 to 1.0. The sieving coefficient is different for different solutes and also depends on membrane characteristics. Convective transport markedly increases the removal of those middle-and large-sized molecules that are poorly cleared by diffusion-based therapies.
II. BASICS OF HEMODIAFILTRATION. HDF is a “hybrid” therapy combining within the same dialyzer module the two main solute transport mechanisms described above: diffusion and convection.
A. Clearance due to diffusion and convection in HDF. The total clearance results from the sum of diffusive and convective clearances. For detailed equations, see Table 17.1. Convective clearance for a given solute depends on the total ultrafiltered volume and the solute sieving coefficient for the membrane being used. The total ultrafiltered volume is the sum of the fluid removed during the treatment for the purpose of correcting extracellular fluid overload plus the volume of “replacement or substitution fluid” infused during the treatment for the purpose of enhancing convection.
Formulas for Solute Clearance with Hemodiafiltration | |
The EUDIAL group (Tattersall, 2013) has proposed the following equations to describe clearance with HDF.
The diffusive component (KD) can be estimated as follows:
![]()
where Qb is blood flow rate; Qd is dialysis fluid flow rate; and KoA is solute-specific dialyzer mass transfer-area coefficient.
The convective component (KC) can be estimated as follows:
![]()
where Qf is the convective flow rate and S is sieving coefficient.
The total clearance KT can be estimated as the sum of both components as follows:
KT = (KD + KC) × DF
in which DF stands for dilution factor, depending on how the replacement fluid is infused during the treatment (postdilution, predilution, or mixed dilution).
B. Substitution mode: Postdilution, predilution, and mixed dilution. Infusing the substitution fluid solution in postdilution mode (Fig. 17.1) means that the fluid is added to the blood flow stream as it exits the hemodialyzer. This is the most efficient option for solute clearances (Fig. 17.1) because there is no dilution of blood in the dialyzer, where convection is occurring. However, when blood flow rate is limited, or when adverse hemorheological conditions (such as high hemoglobin or high protein concentration) are present, or when the rate of substitution fluid infusion needs to be quite high, to avoid hemoconcentration in the filter, one can infuse all of the replacement fluid (predilution mode, Fig. 17.2) or a part of it (mixed dilution, Fig. 17.3) into the blood line upstream to the filter (Pedrini, 2003). The predilution and mixed-dilution modes reduce solute clearance considerably because the concentration of toxins in the blood entering the dialyzer is diluted by the substitution fluid. Advantages and disadvantages of each substitution fluid infusion mode are given in Table 17.2.
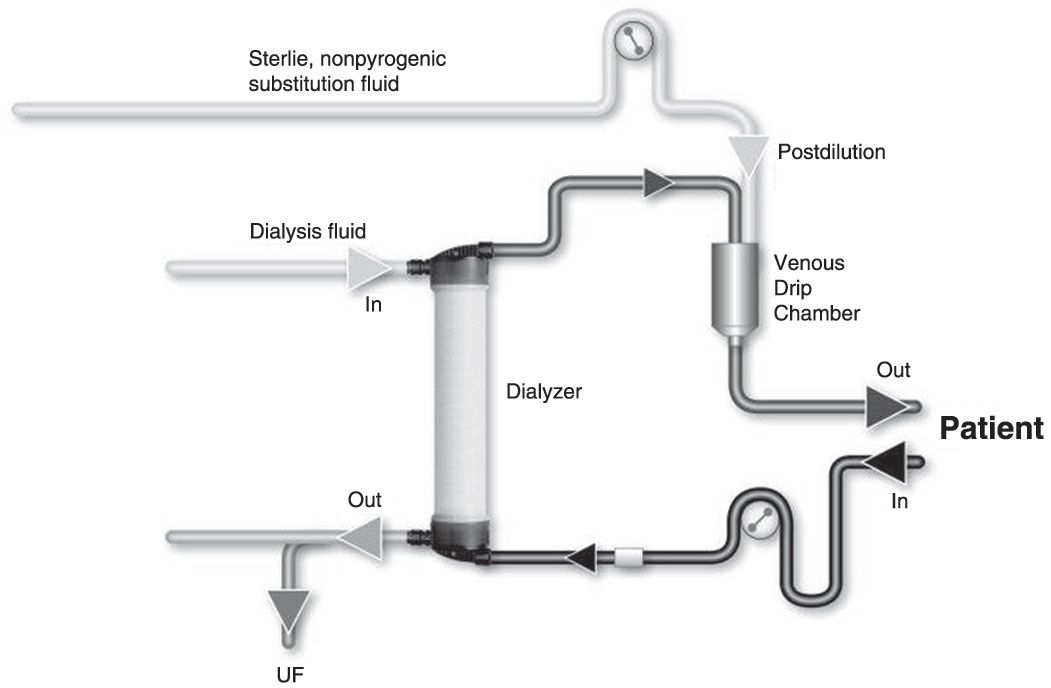
FIGURE 17.1 Postdilution online hemodiafiltration.
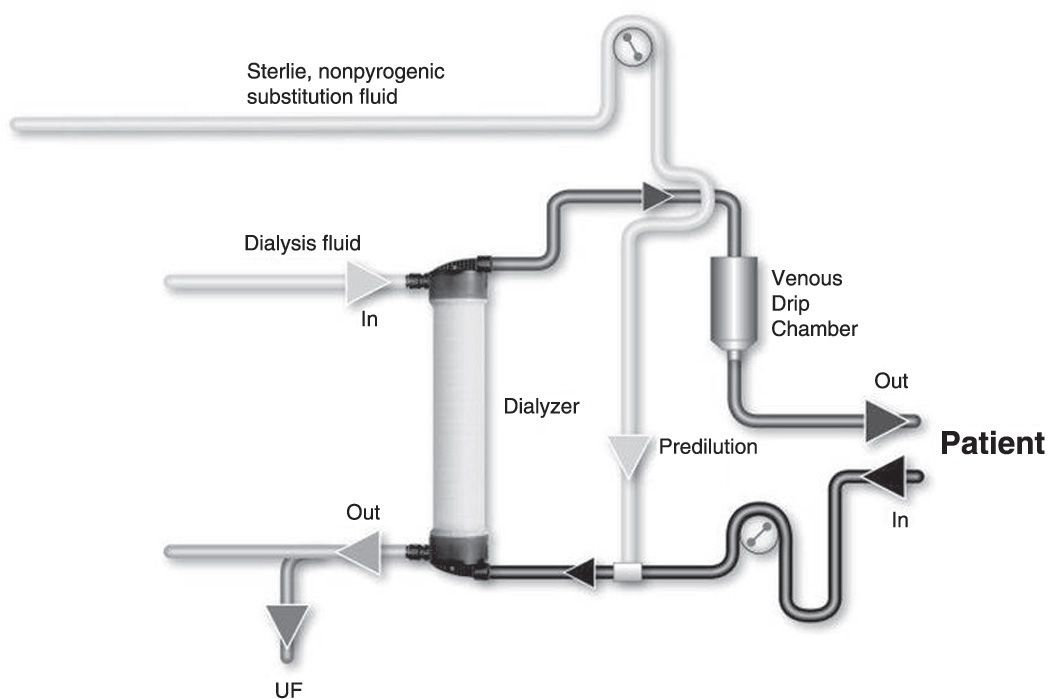
FIGURE 17.2 Predilution online hemodiafiltration.
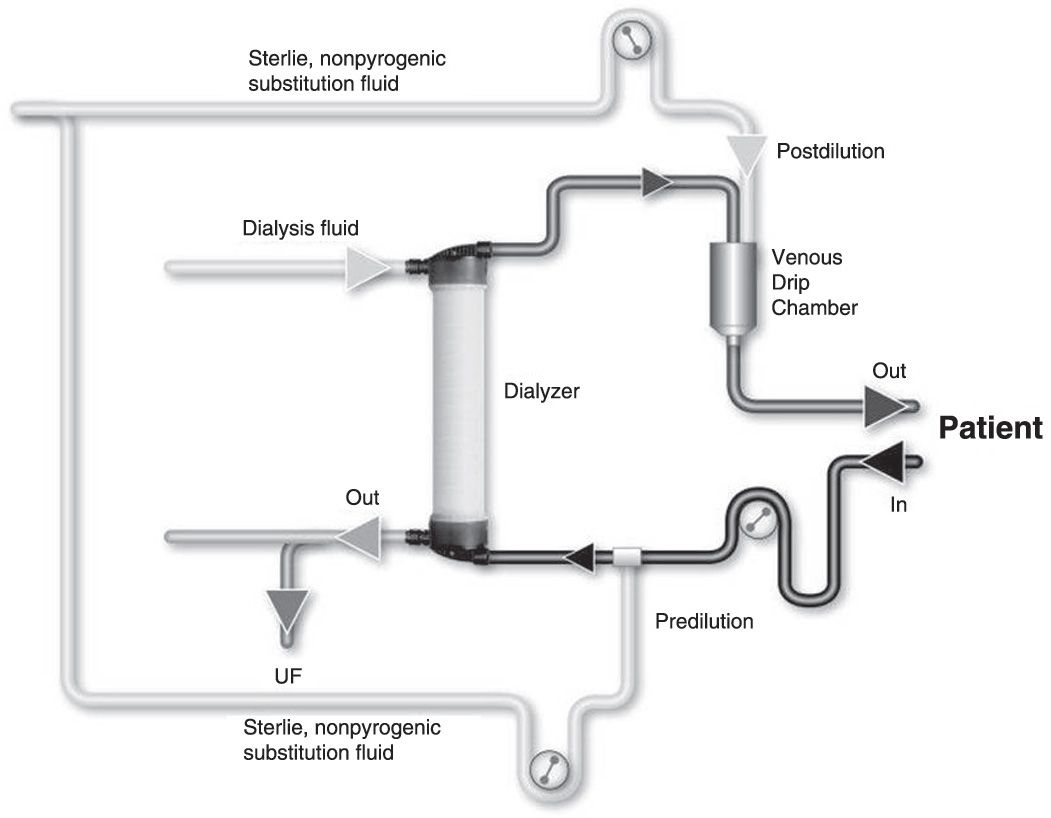
FIGURE 17.3 Mixed-dilution online hemodiafiltration.
Advantages and Shortcomings of Each of HDF Modalities | |
Postdilution | Predilution | Mixed Dilution |
Pros | ||
• High solute clearance and removal of small-, middle-, and high-molecular-weight solutes • Reduced volume of substitution fluid relative to the other modalities | • Hemodilution – Decrease in proteincrit and hematocrit – Reduced viscosity and oncotic pressure – Reduced fiber and membrane fouling – Permits HDF with suboptimal blood flow or unfavorable hemorheological conditions • Facilitates protein-bound solute clearance and removal • Preserves hydraulic and solute membrane permeability (reduces membrane stress) | • Avoids drawbacks of both post-and predilution methods • Permits HDF under suboptimal blood flow conditions and unfavorable hemorheological conditions |
Cons | ||
• Hemoconcentration – Increase in proteincrit and hematocrit – Increase in viscosity and oncotic pressure – Potential membrane fouling • Reduced hydraulic and solute membrane permeability – Increase in transmembrane pressure – Reduced sieving coefficient – Fiber clotting – Potential alarms – Increased membrane stress – Potential albumin leakage | • Reduced solute clearance and removal of small-, middle-, and high-molecular-weight solutes • Increased volume of substitution fluid needed (twofold) | • Require specific hardware equipment – Two infusion pumps – Specific blood tubing set • Requires specific software and algorithm – Accounting for hematocrit and proteincrit changes – Adjusting post-/preinfusion ratio, keeping transmembrane pressure in target range – Increased volume of substitution fluid (only 1.3-fold) |
C. Technical issues
1. Vascular access. Patients treated with HDF require a vascular access capable of delivering an extracorporeal blood flow of at least 350–400 mL/min on a reliable basis.
2. High-flux hemodiafilter. The semipermeable membrane should have a high hydraulic permeability (KUF >50 mL per hour per mm Hg), high solute permeability (sieving coefficient for β2-microglobulin >0.6), and an optimal surface area of exchange (1.60–1.80 m2). Further, low internal blood resistance (internal fiber diameter >200 micrometers, sufficient number of fibers; length of fiber bundle <30 cm) is highly desirable to reduce hemoconcentration and facilitate ultrafiltration.
3. Online production of substitution fluid. Outside of the United States, most dialysis machine manufacturers provide an upgrade option enabling direct production of substitution fluid for intravenous infusion from dialysis solution (Blankestijn, 2010). Such online techniques enable provision of virtually unlimited amounts of sterile nonpyrogenic substitution fluid at a relatively low cost. All studies have shown that online production of substitution fluid is a safe, reliable, and economically viable solution for routine clinical application (Canaud, 2000). This approach has gained the approval of all European regulatory bodies operating under the European Community label.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





