Hematologic Abnormalities
Steven Fishbane
Hitesh H. Shah
I. ANEMIA
A. Etiology. The anemia of chronic kidney disease (CKD) is primarily due to insufficient production of the glycoprotein hormone erythropoietin (EPO). Although EPO can be produced in many of the body’s tissues, EPO required for erythropoiesis generally is produced by endothelial cells in proximity to the renal tubules. As renal excretory function is lost, there is a relative decline in the production of EPO that correlates with the declining glomerular filtration rate. The severity of the resulting anemia varies, but if untreated, then hematocrit values in end-stage kidney disease (ESKD) of 18%-24% are typical. While the primacy of EPO deficiency is indisputable, other factors may play important contributory roles. Also, patients with ESKD may develop any of the other causes of anemia common in nonuremic subjects.
B. Consequences of anemia
1. Symptoms. The manifestations of anemia may be due both to the effects of decreased oxygen delivery to tissues and to the heart’s compensatory changes. The most prominent symptoms of anemia are fatigue and dyspnea. Symptoms develop slowly, and the patient may gradually constrict his or her activities in compensation. The patient’s overall sense of well-being is diminished. Other symptoms may include difficulty concentrating, dizziness, sleep disorders, cold intolerance, and headaches. The heart responds to diminished oxygen-carrying capacity of blood by attempting to maintain systemic oxygen delivery with increased cardiac output and left ventricular hypertrophy. Patients may notice worsening dyspnea and palpitations at this stage. Other problems include deranged hemostatic function, impaired immune function, and diminished cognitive and sexual function. Exacerbations of angina, claudication, and transient ischemic attacks may also be observed.
2. Physical examination. The primary physical examination finding of anemia is pallor, which may be best detected on the palms of the hands, the nail beds, and the oral mucosa. A systolic ejection murmur due to increased cardiac flow may be heard over the precordium.
C. Treatment
1. Medications. Because EPO deficiency is the primary cause of anemia in patients with CKD, agents that replace erythropoietin have a primary role in treatment. Since the last edition of this handbook, the preferred term for these agents has changed to erythropoiesis-stimulating agents (ESA). Drugs may be erythropoietin analogs or may stimulate erythropoiesis in other ways. There are many different erythropoietin analogs commercially available in the United States and elsewhere: Epoetin alfa (Epogen, Procrit) and darbepoetin alfa (Aranesp) are currently available in the United States, and methoxy-polyethyleneglycol-epoetin beta (Mircera) is widely used in Europe and probably will be available shortly in the United States. Peginesatide (Omontys) currently is not being marketed in the United States, after a substantial number of allergic reactions occurred with its use. The cause is currently being investigated. Epoetin alfa is a glycoprotein that is indistinguishable from native erythropoietin. It is manufactured by recombinant DNA technology and has a molecular weight of 30,400 Da and a circulating half-life after intravenous administration of approximately 8 hours. Darbepoetin alfa is a synthetic analog of erythropoietin with increased carbohydrate content that increases the molecular weight by approximately 20% compared with native erythropoietin. As a result of the altered structure, the drug’s pharmacokinetics are changed and the serum half-life is increased to approximately three times longer, 24 hours, compared with epoetin alfa. Mircera has an unusually long serum half-life of approximately 5.5 days. Peginesatide is a synthetic peptide attached to polyethylene glycol that mimics the structure of erythropoietin, but that has no amino acid sequence homology to EPO. Biological analogs of ESAs, so-called biosimilars, have been manufactured and are in use outside of the United States. The safety of these agents has been variable, but under careful FDA scrutiny, it is likely that biosimilar forms of EPO will become available in the United States.
One new class of ESAs currently under development acts to stabilize hypoxia inducible factor-1 (HIF). Synthesis of HIF is increased in the presence of hypoxia, and HIF acts to increase the transcription of EPO. HIF is rapidly degraded when normoxic conditions are present, and drugs that stabilize HIF result in increased endogenous erythropoietin production, even in anephric individuals. These drugs will be an important new class of ESAs if they are demonstrated to be safe and effective.
2. Benefits of anemia treatment with ESA.
a. Effect on outcomes. Cross-sectional and retrospective studies have suggested that anemia in patients undergoing hemodialysis is associated with increased mortality, particularly when the hemoglobin concentration is <10 g/dL (100 g/L). Analyses of large administrative
and clinical databases have shown that risk for mortality, hospitalization rate, and hospitalization days continue to decrease even at hemoglobin levels >11 g/dL (110 g/L). In contrast to these observational studies, interventional studies have not demonstrated improved outcomes following normalization of hemoglobin with ESA treatment. In fact, cardiovascular outcomes in these studies generally have been worse (see below).
and clinical databases have shown that risk for mortality, hospitalization rate, and hospitalization days continue to decrease even at hemoglobin levels >11 g/dL (110 g/L). In contrast to these observational studies, interventional studies have not demonstrated improved outcomes following normalization of hemoglobin with ESA treatment. In fact, cardiovascular outcomes in these studies generally have been worse (see below).
b. Reduction in transfusion-related complications. Prior to ESA therapy, up to 20% of patients on dialysis required frequent transfusions with attendant risk of immediate transfusion reactions, viral infection, iron overload, and immune sensitization. The rate of blood transfusion has been greatly reduced by the use of ESA therapy.
c. Improved quality of life and overall sense of well-being. Various assessment tools have documented an improved quality of life and functional status in ESKD patients treated with ESA. Patients feel less fatigued and their exercise capacity increases. Symptoms that had been disabling in the pre-ESA era are now easily managed. However, the target level of hemoglobin for optimized quality of life is not completely known. Whether higher hemoglobin targets further improve quality of life is unclear. Some studies suggest that improvements may continue as hemoglobin is raised toward the normal range, while others have found no improvement in quality of life despite higher hemoglobin targets.
3. Risks of ESA Therapy. Several well-powered randomized controlled trials have tested the safety of ESA treatment aiming at relatively high hemoglobin targets (13-15 g/dL, or 130-150 g/L) in patients with CKD. In these studies, the comparison groups (controls) were either given ESA treatment to a lower Hgb target or, in one study, the control group received mostly placebo. Four such trials are particularly noteworthy: the Normal Hematocrit Trial (Besarab, 1998), CREATE (Drueke, 2006), CHOIR (Singh, 2006), and TREAT (Pfeffer, 2009). Only one of these four studies (Besarab, 1998) was done in dialysis patients, while the other three recruited nondialysis CKD subjects with eGFR or CrCl normalized to 1.73 m2 in the range of 15-35 mL/min (CREATE), 15-50 (CHOIR), or 20-60 (TREAT). While results were somewhat inconsistent, there was a strong general trend toward increased cardiovascular risk, including risk of death, with ESA treatment to such high Hgb targets.
The mechanism of harm for an ESA treatment with a Hgb target >13 g/dL is unknown. The benefit versus risk of ESA treatment aiming at a lower Hgb target has not been formally studied in a randomized fashion. Post hoc analysis of these high Hgb target studies suggests that a higher achieved Hgb level per se may not be the source of
increased risk. In these trials, mortality was higher in those patients receiving high doses of ESAs; however, it is not at all clear whether this association was causal. Patients requiring high doses of ESAs, or so-called ESA-resistant patients, show many markers of increased illness severity such as cachexia and increased levels of serum inflammatory markers, and ESA resistance is associated with a poor prognosis for survival. In one of the randomized trials noted above (TREAT), the risk of stroke was doubled, and the risk of cancer was also increased in the group given an ESA targeting a high Hgb level. The results of these studies prompted the FDA to include “black box” warnings in the product inserts for ESAs, and for various guideline committees to revise target Hgb levels downward, with the idea that one should use ESAs sparingly, and attempt only partial correction of anemia.
increased risk. In these trials, mortality was higher in those patients receiving high doses of ESAs; however, it is not at all clear whether this association was causal. Patients requiring high doses of ESAs, or so-called ESA-resistant patients, show many markers of increased illness severity such as cachexia and increased levels of serum inflammatory markers, and ESA resistance is associated with a poor prognosis for survival. In one of the randomized trials noted above (TREAT), the risk of stroke was doubled, and the risk of cancer was also increased in the group given an ESA targeting a high Hgb level. The results of these studies prompted the FDA to include “black box” warnings in the product inserts for ESAs, and for various guideline committees to revise target Hgb levels downward, with the idea that one should use ESAs sparingly, and attempt only partial correction of anemia.
4. Indications for ESA therapy and target hemoglobin. ESA therapy should generally be initiated in CKD patients when the Hgb falls below 10 g/dL (100 g/L). The optimal Hgb level for a patient with ESKD is not known. The Kidney Disease: Improving Global Outcomes (KDIGO) anemia guidelines (2012) simply recommend that Hgb for dialysis patients should not exceed >11.5 g/dL (115 g/L). This recommendation is in some conflict with current FDA prescribing instructions, which recommend holding ESA dosing when the hemoglobin is >11.0 g/dL (110 g/L). A reasonable hemoglobin target for patients on dialysis would be 9.5-11.5 g/dL (95-115 g/L).
a. Effect of volume status on target hemoglobin. When Hgb is assessed prior to a hemodialysis session, extracellular volume tends to be high, and so due to dilution, the Hgb level is at a relatively low value for the week. Monday/Tuesday predialysis Hgb levels are at their low point for the week and are about 0.3 g/dL (3 g/L) lower than midweek predialysis levels. Immediate postdialysis Hgb levels can be very substantially higher than predialysis values. So the time-averaged weekly Hgb value can be substantially underestimated by predialysis numbers. In patients with markedly fluctuating degrees of fluid overload, a change in predialysis Hgb may reflect a change in fluid status more than a change in red cell mass. This potential dilution issue must be kept in mind when monitoring Hgb levels and using this information to adjust ESA dose. For the same reason, a change from a 3 per week to a more frequent dialysis schedule can result in a modest increase in Hgb that is more a reflection of reduced extracellular fluid volume and sampling after a 1-day interdialytic interval rather than to an increase in red cell mass. Lastly, if one is trying to parse Hgb targets from nondialysis CKD patients to patients receiving dialysis, a Hgb target of 11 g/dL (110 g/L) in CKD may
correspond to a somewhat lower predialysis target value in dialysis patients due to the dilution effect.
correspond to a somewhat lower predialysis target value in dialysis patients due to the dilution effect.
5. Route of administration
a. Subcutaneous versus intravenous ESAs. The subcutaneous route improves the efficiency of therapy, resulting in a reduced dosing requirement (of about 25%) for shortacting ESAs, specifically epoetin alfa. (Kaufman, 1998). When epoetin is given intravenously, its short half-life probably results in some drug never binding to erythropoietin receptors prior to clearance of epoetin from the circulation. When given subcutaneously, the serum halflife of epoetin is extended, allowing for more efficient receptor binding and a greater erythropoietic effect. Despite the dose reduction advantage of subcutaneous administration, the majority of patients undergoing hemodialysis in the United States continue to be treated via the intravenous route. The primary reason is probably discomfort with subcutaneous injections, whereas reduced dosing requirements are a benefit that does not directly accrue to the patient. ESAs with a longer serum half-life provide extended time in circulation to allow for greater opportunity for the drug to bind to erythropoietin receptors. There is probably no advantage or need for subcutaneous dosing for methoxypolyethylene glycol-epoetin beta, peginesatide or possibly even darbepoetin alfa. Intravenous administration of any of these agents would appear to be a better choice than subcutaneous injection of epoetin alfa in hemodialysis patients, to reduce patient discomfort. For patients on peritoneal dialysis, subcutaneous injections remain the dominant route of administration.
6. Dosing
a. Initial dose. Treatment with ESA should ideally be initiated, if required, in the pre-ESKD period. If treatment needs to be initiated for a patient already on dialysis, reasonable starting doses of epoetin alfa for a hemodialysis patient would be 2,000-3,000 units three times per week, and for a peritoneal dialysis patient 6,000 units once per week. A typical dose of darbepoetin alfa would be approximately 25 mcg once weekly for a hemodialysis patient or 60 mcg every 2 weeks for a patient on peritoneal dialysis. A typical dose of Mircera would be 150 mcg given once monthly. Selection of a specific dose requires clinical judgment as to how symptomatic the patient is and the starting level of hemoglobin. An excessively rapid rise in the hemoglobin level should be avoided, as this may lead to an increased risk of worsening hypertension.
b. Initial response and plateau effect. During the initiation phase of therapy, hemoglobin should be checked every 1-2 weeks, and the ESA dose adjusted as needed. It is
very common during the initiation of treatment for a “plateauing” of effect to occur; either the hemoglobin stops increasing, or escalating doses of ESA are required to reach therapeutic targets. This period of blunted response is often due to the development of iron deficiency. Once the target level of hemoglobin has been reached, the hemoglobin should be checked every 2-4 weeks. During this maintenance phase of therapy, the dose of ESA should be adjusted on the basis of subsequent changes in hemoglobin (Fig. 34.1).
very common during the initiation of treatment for a “plateauing” of effect to occur; either the hemoglobin stops increasing, or escalating doses of ESA are required to reach therapeutic targets. This period of blunted response is often due to the development of iron deficiency. Once the target level of hemoglobin has been reached, the hemoglobin should be checked every 2-4 weeks. During this maintenance phase of therapy, the dose of ESA should be adjusted on the basis of subsequent changes in hemoglobin (Fig. 34.1).
The patient’s responsiveness to ESA should be reassessed on a continuing basis. Most patients will be responsive, with hemoglobin values consistently >10 g/dL (100 g/L), and an epoetin dose of <5,000 units three times per week. In contrast, some patients will have or develop relative resistance to therapy. These patients need to be fully evaluated for causes of ESA hyporesponse. The responsiveness to ESA in all patients should be evaluated on an ongoing basis because the degree of responsiveness changes over time. In our experience, the development of resistance often signals the presence of iron deficiency or infection.
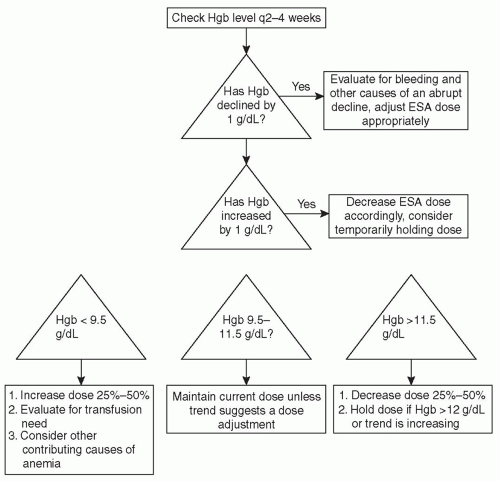 FIGURE 34.1 Flow chart for adjusting the ESA dose based on hemoglobin (Hgb) results for dialysis patients. |
Data from ESA practice patterns in the United States suggest that the median weekly dose of intravenous EPO is about 7,000 units per week, and for darbepoetin this is 25 mcg per week (Coritsidis, 2014). Side effects of ESA therapy have been reported in patients getting the high doses. ESA-resistant patients are a selected group with poor outcome; however, it is possible that use of high doses of ESAs per se may be associated with increased side effects. Response to ESAs tends to plateau at high doses, and use of very high doses is uneconomical. For these reasons, the 2012 KDIGO guidelines recommend not generally exceeding four times the usual baseline weight-adjusted dose of EPO when managing EPO-resistant patients (KDIGO Anemia, 2012).
c. Individualized anemia management. The pharmacodynamics of ESA use are complicated, as the achieved Hgb levels depend not only on ESA sensitivity, but on the average lifespan of red blood cells (RBCs) in a given patient. A number of algorithms have been developed with the goal of maximizing the time that Hgb remains in a desired range. Algorithms in development may be enhanced by estimates of Hgb measured during each dialysis session by use of optical or ultrasound blood line sensors. The use of such algorithms has been reported to reduce Hgb variability as well as overall ESA dose (Lines, 2012; Gaweda, 2014).
D. Side effects of ESA therapy. See Section 3, above, for a discussion of cardiovascular risks of ESA therapy.
1. Worsening of hypertension. This is a common problem during the partial correction of anemia with ESA therapy. In some patients, there will be a need to increase antihypertensive medication doses. However, it is rare for ESA to be withdrawn because of uncontrollable hypertension. Risk factors include preexisting hypertension, a rapid increase in hemoglobin, the presence of dysfunctional native kidneys, and severe anemia prior to treatment. The cause of the hypertensive effect is incompletely understood. Factors that may contribute include the partial reversal of hypoxic vasodilation as the hemoglobin rises, reduced nitric oxide, increased cytosolic calcium levels, increased plasma endothelin levels, activation of the renin-angiotensin-aldosterone system and others. Various antihypertensives, including long-acting calcium channel blockers, are effective for treating hypertension associated with ESA.
2. Seizures. These may occur in a small number of patients during periods of rapidly increasing hemoglobin in association with hypertension. The risk of seizures is small using current ESA dosing protocols.
3. Graft clotting. The increase in blood viscosity with higher hemoglobin values from either ESA therapy or other causes could theoretically cause increased dialyzer and arteriovenous
graft clotting. Studies to date have not consistently demonstrated an increased risk of thrombosis when the hemoglobin is raised to the 11-12 g/dL (110-120 g/L) range. The impact of higher hemoglobin levels is controversial. It should be clear that some patients may experience substantial hemoconcentration during or after the hemodialysis treatment, and effects on blood viscosity and risk for access thrombosis may be a particular concern in this setting.
graft clotting. Studies to date have not consistently demonstrated an increased risk of thrombosis when the hemoglobin is raised to the 11-12 g/dL (110-120 g/L) range. The impact of higher hemoglobin levels is controversial. It should be clear that some patients may experience substantial hemoconcentration during or after the hemodialysis treatment, and effects on blood viscosity and risk for access thrombosis may be a particular concern in this setting.
4. Stroke. The risk of stroke has been increased in some of the randomized trials of ESAs where a relatively high Hgb level has been targeted, but this was not noted in all such studies.
5. Effect on Kt/V. During dialysis, urea is removed from both red cells and plasma, and so urea clearance and Kt/V-urea are not affected by an increase in the Hgb. Creatinine and phosphorus are removed from the plasma only during passage of blood through the dialyzer, and as the Hgb is increased, at any given blood flow rate, the plasma flow rate and creatinine and phosphorus clearances will be proportionately reduced.
E. ESA Treatment and Cancer. In studies of ESA treatment for anemia related to chemotherapy or cancer, there has been some evidence to suggest that ESA treatment could reduce overall and progression-free survival. This has led to significant changes in the approach to ESA treatment in patients with cancer. Because some patients with ESKD may have either an active or past malignancy, the subject is relevant and affects treatment decisions (Hazzan, 2014).
The data, however, are not entirely consistent. For example, five published meta-analyses of published trials have not found ESA treatment to adversely impact complete responses, disease progression, or progression-free survival. However, specific studies of certain types of cancer do indicate an adverse effect, for example, in patients with head and neck cancer receiving radiotherapy. It should be noted that in studies demonstrating potential harm, ESA treatment was used to target relatively high hemoglobin levels (up to 16 g/dL in men). While the meta-analyses provide some reassurance, the adverse effects of ESAs in some studies should drive a conservative approach to treatment until the question is fully resolved.
We would suggest that for ESKD patients with a history of past malignancy, ESA therapy can be cautiously employed with hemoglobin targets as noted above for general treatment of ESKD patients. In patients with active malignancy, whether or not the patient is currently receiving chemotherapy, we would recommend a more conservative approach to treatment. This is based on an uncertainty in current knowledge on progression-free survival and increased thromboembolic risk in cancer patients. We would suggest lowering the target hemoglobin to 9-10 g/dL (90-100 g/L). For symptomatic, urgent anemia correction, blood transfusion should be employed.
F. Causes of decreased response to ESA therapy
1. Iron deficiency. The most important cause of a suboptimal response to ESA therapy is iron deficiency. Iron deficiency can be present at the outset of therapy, but more commonly, it develops during therapy, either due to rapid utilization of iron to support erythropoiesis or as the result of blood loss (Table 34.1).
a. Blood loss. Hemodialysis patients develop iron deficiency primarily because of chronic blood loss. Between retention of blood in the dialysis lines and filter, surgical blood loss, accidental bleeding from the access, blood sampling for laboratory testing, and occult gastrointestinal bleeding, iron losses may be substantial. Because of the overall burden of blood loss, it is very difficult to maintain iron stores in hemodialysis patients using oral iron supplements only. Losses in peritoneal dialysis patients are substantially less, and these patients can often be maintained on oral iron therapy.
b. Functional iron deficiency. In addition to a depleted iron supply, the demand for iron increases during ESA treatment, leading to a further strain on depleted iron stores. After the intravenous injection of ESA, there is an increase in the rate of erythropoiesis that leads to a greater immediate need for iron. In this setting, iron deficiency may occur even in the face of normal body iron stores. This phenomenon has been termed “functional iron deficiency.”
c. Inflammation (reticuloendothelial blockade). Occult inflammation is often present in ESKD patients. It causes an increase in serum hepcidin concentrations, which
causes reduced intestinal iron absorption and diminished availability of iron in storage tissues.
causes reduced intestinal iron absorption and diminished availability of iron in storage tissues.
TABLE 34.1 Causes of Iron Deficiency in Hemodialysis Patients | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
d. Poor absorption of dietary iron. Iron deficiency among patients on dialysis may be exacerbated by poor absorption of dietary or medicinal iron. However, the subject is controversial, and results from studies have been conflicting.
2. Diagnosis






