Fig. 12.1
H. pylori-associated factors which enable colonization and persistence [6]. Initially, H. pylori produces urease which raises the pH through ammonia production. Bacterial helical rod shape and flagellar-based motility help intrusion of bacteria to niche, away from acid lumen. Different variably expressed adhesins (like SabA, BabA) may shift the balance to cell-associated bacteria. Cell-associated bacteria may alter gastric epithelial cell through VacA, CagA, and CagL which all have multiple cellular targets. This includes disruption of cell polarity, induction of chemokines and/or the gastric hormone gastrin, and inhibition of acid secretion. T4SS Cag type IV secretion system, PS phosphatidylserine, α5β1 and αvβ5 integrin subunits, VacA vacuolating cytotoxin A, CagA cytotoxin-associated gene A protein, and CagL cytotoxinassociated gene L protein. (Reprinted by permission from Macmillan Publishers Ltd, Nature Publishing Group, Ref. [6])
The interaction between bacteria and host is very complex and not fully elucidated and includes different virulence factors involved into to pathogenesis [6].
The majority of the H. pylori-infected population remains asymptomatic, but some individuals may develop chronic gastritis , peptic ulcer, MALT lymphoma, or carcinoma [20]. Factors determining the subset of infected individuals developing the disease compared with asymptomatic H. pylori carriers remain unclear. It is now becoming increasingly clear that the H. pylori virulence factors as well as host immune factors contribute closely to those differences in H. pylori pathogenicity .
Epidemiology and Transmission
Over the past decades, the prevalence of H. pylori in the developed world steadily decreases. Most recent international studies have shown that H. pylori prevalence varies between countries, with lowest prevalence of 5–10 % in Western Europe and the USA and very high prevalence in developing countries of even 80 % [21, 22]. An increased prevalence in developing countries is mainly due to the combined effects of poor living conditions, poor hygiene, and overcrowding [23]. In developing countries, H. pylori infection is acquired predominantly during early childhood; however, in the more developed areas the infection gradually increases with age with the highest incidence rate in childhood and adolescence [24, 25].
The modes of H. pylori transmission are still not entirely clarified, but person-to-person contact is the most commonly implicated mechanism through oral–oral, fecal–oral, or gastric–oral route [26]. It has been described that transmission primarily occurs between mothers and their offspring or between siblings [25, 27]. Currently available evidence indicate that especially in populations with low H. pylori prevalence the infected mother is likely to be the main source for childhood H. pylori infection [27]. However, only few studies have longitudinally examined factors which influence H. pylori transmission. In the well-designed longitudinal study, Muhsen et al. included Israeli Arab children aged 3–5 years from three villages in northern Israel which were followed-up for 3–4 years [28]. Having H. pylori-infected sibling was identified as an independent risk factor for both early and persistent H. pylori infection. It was also shown that persistent H. pylori infection in older siblings always precedes infection in younger siblings especially if the age difference was less than 3 years [29].
Due to very close interpersonal contact between children in day-care centers, it was proposed that their attendance could also be a risk factor [24]. However, a meta-analysis of 16 studies did not confirm this hypothesis, but included studies substantially varied in the methodology; they were performed in a different types of childcare, in different age groups, and with different exposure duration, which all resulted in a high heterogeneity in the meta-analysis [24].
There is some evidence of positive correlation between presence of H. pylori in the oral cavity and gastric mucosa [30]. Moreover, it seems that eradication rate is significantly higher in stomach than in oral cavity. These results suggest that infected dental plaque, saliva, or other places in the oral cavity may be a source for infection and reinfection of H. pylori through oral–oral route [30]. Presence of H. pylori in tonsils and its impact on infection transmission is still controversial [22].
Gastritis
Gastritis is defined as histologically confirmed inflammation of the gastric mucosa mainly composed of lymphocytes, plasma cells, histiocytes, and granulocytes within the lamina propria [31]. H. pylori is by far the most common etiological agent causing gastritis in adults and children. The most common site of infection is the antrum, which is an absorptive rather than a secretory region of the stomach enabling the slightly higher pH at which the organism can survive [5]. In the initial phase of H. pylori infection, the gastric mucosa becomes acutely inflamed with impairment in the acid secretion [32]. In large majority of patients, gastritis progresses to chronic active gastritis which is characterized by the presence of both mononuclear and neutrophilic (“active”) inflammation [31]. In children, the “active” or neutrophilic count is lower than that reported in adults.
In adults, H. pylori infection causes three main gastritis types: mild pangastritis where inflammation evenly affects the whole stomach; antrum-predominant gastritis where the degree of inflammation is strongest in the antrum and acid secretion tends to be increased; and the most infrequent phenotype affecting only approximately 1 % of the infected subjects’ body-predominant gastritis with even atrophic changes and impaired gastric acid secretion [32]. Antrum-predominant gastritis may subsequently be complicated by duodenal ulcer while corpus-predominant atrophic gastritis increases the risk of gastric cancer [33]. Whether this distinction between gastritis types exist already in children or it develops later in life is still unknown [34].
In addition to the topographic expression of H. pylori-induced chronic gastritis , the characterization of gastritis needs to include report on the activity and chronicity of inflammation and on the development of atrophic changes with or without intestinal metaplasia [35].
H. pylori gastritis has unique features in children, such as the nodular aspect of the gastric antrum, antral predominance of gastritis in most patients, and uncommon diagnosis of gastric atrophy and intestinal metaplasia. Nodular gastritis is usually characterized as an endoscopic appearance which has been described as gooseflesh-like or cobblestone markings on the gastric mucosa (Fig. 12.2) [36]. Antral nodularity seen on endoscopy is histologically associated with inflammatory cell infiltrates and lymphoid follicles, and it is the only sign with a high positive predictive value for the presence of H. pylori infection [37].
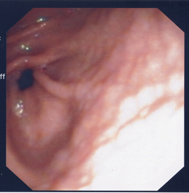

Fig. 12.2
Antral nodularity associated with H. pylori infection
After successful eradication therapy, the neutrophils quickly disappear and any persistence of neutrophils and/or mononuclear infiltrate could be an indication of the treatment failure.
Peptic Ulcer Disease
Gastritis and peptic ulcer disease were previously considered as separate diseases; however, better understanding of the pathomechanisms and discovery of H. pylori revealed the close relation between those two entities . Peptic ulceration of the stomach or duodenum, either primary or secondary, is almost always accompanied by abnormalities of the gastric mucosa, either a gastritis or a gastropathy [38]. Peptic ulcer disease is the ultimate loss of mucosal integrity and develops when the protective mechanisms of the gastrointestinal mucosa, such as mucus and bicarbonate secretion, are overwhelmed by the damaging effects of gastric acid and pepsin [39]. By the definition, peptic ulcers are deep mucosal lesions that disrupt muscularis mucosa layer of gastric or duodenal wall. They occur in the stomach mainly in antrocorporeal mucosal transitional zone along the lesser curve or proximal duodenum, duodenal bulb [40]. Peptic ulcers are more common in duodenum than in stomach and H. pylori is the most frequent cause . Other etiological factors associated with gastritis and peptic ulcer disease are presented in Table 12.1.
Table 12.1
Causes of gastritis and peptic ulcer disease
Primary | H. pylori associated |
H. pylori negative (idiopathic) | |
Hypersecretory states | |
Zollinger–Ellison syndrome | |
G cell hyperplasia/hyperfunction | |
Systemic mastocytosis | |
Cystic fibrosis | |
Hyperparathyroidism | |
Short bowel syndrome | |
Renal failure | |
Secondary | Stress ulcers |
Drugs | |
Aspirin | |
Nonsteroidal anti-inflammatory drugs (NSAID) | |
Chorticosteroids | |
Chemotherapy | |
Valproic acid | |
Alcohol | |
Potassium chloride | |
Immune/allergic | |
Allergic gastritis and eosinophilic gastritis | |
Graft-versus-host disease | |
Henoch–Schönlein gastritis | |
Coeliac disease | |
Autoimmune disease | |
Granulomatous gastritides | |
Crohn’s disease | |
Foreign body reaction | |
Idiopathic | |
Sarcoidosis | |
Histiocytosis X | |
Tuberculosis | |
Menetrier disease | |
Other infections | |
Helicobacter heilmanii | |
Cytomegalovirus | |
Phlegmonous gastritis and emphysematous | |
Herpes simplex | |
Influenza A | |
Syphilis | |
Candida albicans | |
Histoplasmosis | |
Mucormycosis | |
Anisakiasis | |
Radiation gastropathy |
Available literature suggests that the lifetime risk for development of peptic ulcer disease in H. pylori-positive patients ranges between 10 and 20 % [41]. Peptic ulcer disease occurs less frequent in children than in adults. A large prospective European multicenter study including more than 1400 symptomatic infected children found gastric or duodenal ulcers in 3.5 % of the children below 6 years of age, in 4.6 % the children aged 6–11 years, and in 10.4 % of those older than 12 years [42]. Subsequent, prospective, also European multicenter study found that even 8.1 % of the children had ulcers or erosions [43]. In a retrospective review of 619 Chinese children who had undergone upper endoscopy for investigation of upper gastrointestinal symptoms, Tam et al. [44] have found peptic ulcers in 6.9 % of the children.
The discovery of H. pylori has turned the pathogenesis of peptic ulcer disease from a hyperacid disease to an infectious and immunopathogenetic disease where a variety of ulcer-promoting host and bacterial factors are involved in the complex pathogenesis [32]. However, despite that, gastric acid secretion remains an important factor of peptic ulcer disease development. Patients with H. pylori gastritis and duodenal ulcers present with a much higher gastric acid output following gastrin stimulation than patients with H. pylori gastritis but without duodenal ulcer [45]. The mechanisms controlling acid secretion are hormonal and very complex in nature . The hormonal drive is exerted by hypergastrinemia, which was originally interpreted to result from the alkalization induced by H. pylori. Higher pH around G cells interrupts the negative feedback control and they continue to release gastrin [32]. Another explanation for hypergastrinemia could be the H. pylori-induced impaired synthesis and release of somatostatin [32]. Somatostatin is a hormone that inhibits gastrin synthesis and subsequently inhibits acid secretion.
Moreover, H. pylori virulence factors also contribute to the development of peptic ulcer disease. In children, as in adults, CagA and VacA genes are the most frequently implicated virulence factors associated with increased risk of peptic ulcer disease [46] .
In gastric ulcer, the role of acid and H. pylori, although certainly contributory, is of less dominance as compared to duodenal ulcer [32].
Epigastric pain or discomfort exacerbated by the meal is often a symptom of peptic ulcer disease in children; however, it may also be a presenting symptom of more common disorders such as non-ulcer dyspepsia and constipation among others [38]. Other presenting symptoms include anorexia, nausea , early satiety, recurrent vomiting, and anemia. Up to 25 % of the children with duodenal ulcers can present silently, approximately 25 % with bleeding and antecedent pain, and the rest with abdominal pain or recurrent vomiting [38, 47]. Among all clinical signs: epigastric tenderness, pain awakening the child at night, hematemesis , melena, and stunted weight gain can be considered as significant risk factors for ulcers or erosions independently [43]. Acute gastrointestinal bleeding is the most common complication of childhood peptic ulcer disease and may occur with longstanding antecedent epigastric pain, whereas a perforated peptic ulcer occurs more rarely [44].
Other Causes of Peptic Ulcer Disease
In adult patients, the second most common cause of peptic ulcer disease are the use of nonsteroidal anti-inflammatory drugs (NSAIDs) [48]. NSAIDs inhibit cyclooxygenase-1 enzyme (COX-1) and reduce prostaglandin synthesis; consequently, diminishing gastric mucosal blood flow and decreasing a production of the mucus–bicarbonate barrier [49]. In children, gastric ulcerations from NSAID ingestion are not nearly as frequent as in adults [38]. When occurs in children, NSAID-induced ulcer is typically presented as hemorrhagic antral gastropathy and ulceration of the incisura. Other drugs associated with drug-induced gastropathy and ulcers are anticoagulants and corticosteroids [50] .
Other very rare causes of peptic ulcer disease in children include hypersecretory states like Zollinger–Ellison syndrome and antral G cell hyperplasia, and should be suspected in children with severe or recurrent duodenal and gastric ulcers, resistant to proton pump inhibitors (PPI) treatment, and in children with multiple ulcers. Other conditions associated with acid hypersecretion are systemic mastocytosis, short bowel syndrome during the first year after surgical resection, hyperparathyroidism, cystic fibrosis, and renal failure [51].
Stress-related ulcers usually occur 24 h after the onset of severe critical illness including shock, hypoxemia, acidosis, sepsis, burns, head injury, encephalopathy, major surgery, and multiple organ system failure [38]. Stress erosions are typically multiple and asymptomatic until cause, sometimes severe, gastointestinal bleeding .
Gastritis and gastric ulcerations are found in 50–60 % of the children with Crohn’s disease [52]. Focally enhanced gastritis is an inflammatory lesion often found in Crohn’s disease and involves discrete inflammatory foci containing lymphocytes, histiocytes, and granulocytes [53]. Focally enhanced gastritis, although in much lower extent, could also be found in ulcerative colitis and indeterminate inflammatory bowel disease .
Ménétrier’s disease is a rare disorder of unknown etiology characterized by enlarged gastric rugal folds. It is a protein-losing gastropathy, clinically presented with nonspecific gastrointestinal symptoms such as nausea , vomiting, anorexia, weight loss, and hypoalbuminemia [54]. Cytomegalovirus and H. pylori are the most frequently found pathogens associated with this condition.
Celiac disease-associated lymphocytic gastritis is characterized by an intense lymphocytosis of the foveolar and surface epithelium and chronic inflammation in the lamina propria. Celiac disease may manifest with dyspeptic symptoms and histological changes which normalize after gluten withdrawal [55, 56] .
Eosinophilic-mediated gastritis may be a presentation of food allergy (allergic gastritis) or be a primary disease (primary eosinophilic gastritis) , as gastric infiltration by eosinophils is a pathological feature found in both conditions. Allergic gastritis mainly affects young infants with cow’s milk protein allergy but multiple food intolerances may also occur. Most common symptoms include vomiting, hematemesis , poor weight gain, or symptoms of gastric emptying [57]. Primary eosinophilic gastritis may manifest at any age and may affect any part of the gastric wall. In mucosal form, children may present with vomiting, abdominal pain, and gastric blood loss; motility disturbances and gastric outlet obstruction may occur if muscular layer is affected [58, 59]. Serosal forms produce eosinophilic ascites and peritonitis [60]. Eosinophilic gastritis may also be a part of the eosinophilic gastroenteritis .
Other causes of primary and secondary gastritis and peptic ulcer disease in children are presented in Table 12.1.
Malignancy
H. pylori is considered the leading cause of gastric cancer worldwide and because of that, in adult population, it is listed as a number one carcinogen. A causal relation between H. pylori infection and the risk of gastric malignancies, including cancer and MALT lymphoma has been clearly proved. However, in children, H. pylori-related malignancy is extremely rare. Evidence increasingly indicates that H. pylori-related gastric carcinogenesis is likely to be the result of a well-choreographed interaction between the pathogen and host, which is dependent on strain-specific bacterial factors, host genotypic traits, and permissive environmental factors [2]. Various factors influence malignant potential including age of infection, bacterial genotype, host immune response, and host genetics. It has been suggested that chronic gastritis, gastric atrophy, intestinal metaplasia, and gastric cancer develop progressively, stepwise over decades, in predisposed individuals infected with H. pylori [61].
Incomplete intestinal metaplasia has been described in the gastric mucosa of H. pylori-infected children, suggesting that it can develop even during childhood and evolve into complete intestinal metaplasia in adults [62]. Moreover, other studies have reported a significant incidence of gastric atrophy (42–55 %) and intestinal metaplasia (13–21 %) in children [63]. Interestingly, a higher incidence of atrophic gastritis has been observed in children from countries with high incidence of gastric cancer [64, 65]. Moreover, current evidence suggests that in high-risk populations the eradication of H. pylori may have the potential to decrease the risk of gastric cancer [66]. There are certain H. pylori genotypes associated with more severe inflammation of gastric mucosa in pediatric patients including CagA, VacA, and BabA, and their detection could be of importance in areas with high risk of carcinoma [67]. In support of this is a report which found that in high-risk population, even children have a high prevalence of H. pylori virulence markers—CagA and VacA [68].
In the pediatric population, there are only a few studies with a small number of patients regarding the association between H. pylori infection and precancerous lesions in both gastric antrum and corpus [63]. There are reports which presented children with H. pylori infection who subsequently developed high-grade B-cell lymphoma which resolved after H. pylori eradication even without chemotherapy [69, 70]. All these data indicate that H. pylori could be associated with malignancies in children; however, the risk seems to be substantially lower than in adults [71].
Clinical Presentation of H. pylori Infection
Recurrent Abdominal Pain
Abdominal complaints including pain, cramps, and nausea are very common in pediatric population. Usually, they are unspecific and can be a symptom of various organic diseases but are very often caused by functional gastrointestinal disorders. Whether H. pylori infection without peptic ulcer disease can cause recurrent abdominal pain remains unclear. Large epidemiological studies found association between recurrent abdominal pain and different social and familial factors like single-parent household, family history of peptic ulcer disease, or functional pain but not to H. pylori status of the child [72]. A performed meta-analysis on the relationship between recurrent abdominal pain and H. pylori infection in children included 38 case control, cross-sectional, and prospective studies and found no evidence for relation between recurrent abdominal pain and H. pylori infection in children [73]. The only pain found associated with H. pylori infection was epigastric pain [73]. Several interventional but uncontrolled studies showed improvement of symptoms after the eradication of H. pylori; however, these studies have several biases; treatment success was not monitored and eradication of the bacteria not reported and in other studies follow-up period was very short, for a few weeks only [74–77]. Based on these results, there is not enough evidence to support a causal relation between H. pylori gastritis and abdominal pain in the absence of peptic ulcer disease. Searching for H. pylori is therefore not recommended in children that otherwise fulfill the criteria of functional abdominal pain, unless upper endoscopy is performed during the diagnostic workup in search for organic disease [4, 67, 78] .
Iron-Deficiency Anemia
Iron-deficiency anemia in children and adolescents has variety of causes and there are still disagreements whether H. pylori infection can be one of them. The potential pathogenesis is reduced iron absorption in infected gastric mucosa, increased iron loss due to microbleeding, and utilization of iron by H. pylori in the stomach [67]. Number of studies suggested that iron deficiency is one of the extragastric manifestations of H. pylori infection in children [79–81]. However, the results of these studies are not unanimous, with most recent studies that reported no such association [82, 83]. This considerable discrepancy in the results is largely due to methodological variation of the studies. Moreover, it is often difficult to distinguish between iron-deficiency anemia due to H. pylori infection and to the other often confounding factors such as poor nutritional status, poor socioeconomic and hygienic conditions, or another underlying disease [4]. Hence, endoscopic examination may be indicated in children with refractory iron-deficiency anemia in order to rule out not only the presence of H. pylori but also other causes of iron-deficiency anemia such as celiac disease or chronic inflammatory diseases [84].
These findings are consistent with clinical guidelines by the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and by the North American Society for Pediatric Gastroenterology Hepatology and Nutrition (NASPGHAN); they recommend that in children with refractory iron-deficiency anemia where other causes have been ruled out, testing for H. pylori infection may be considered [4].
Growth
There are a number of longitudinal studies that support the hypothesis that H. pylori infection might influence growth rate in children [85]; however, none are adequately designed to evaluate confounders of growth including other gastrointestinal infections in childhood, socioeconomic status, living conditions, etc. Despite new information concerning the effect of H. pylori infection on poor growth, the need for well-designed studies remains. Based on published data there is insufficient evidence that H. pylori infection is causally related to growth impairment in childhood [4].
Chronic Idiopathic Thrombocytopenic Purpura
The role of H. pylori in the development of chronic idiopathic thrombocytopenic purpura continues to evolve. H. pylori infection has been proposed to be associated with thrombocytopenic purpura based on a significantly increased platelets count following H. pylori eradication in approximately half of adult patients [86]. Data on children are limited; small case series have been published with conflicting results [87–91]. However, more recent data implicate that eradication of H. pylori could induce a better treatment response in chronic idiopathic thrombocytopenic purpura [92].
Diagnostic Procedures
Based on ESPGHAN/NASPGHAN evidence-based guidelines for H. pylori infection in children testing for H. pylori in children should be performed in properly selected patients (Table 12.2) and with an adequate diagnostic procedures [4]. Current recommendations for children do not approve “test and treat” approach recommended for adults [4]. Therefore, diagnostic procedures should aim to determine underlying disease and not to detect H. pylori.
Table 12.2
Clinical indication for testing for H. pylori infection. (Based on ESPGHAN/ NASPGHAN recommendations Ref. [4])
Justified testing for H. pylori | Gastrointestinal symptoms suggestive of organic disease, serious enough to justify upper endoscopy |
Considered testing for H. pylori | Children with first-degree relatives with gastric cancer |
Children with refractory iron-deficiency anemia when other causes have been ruled out | |
Not justified testing for H. plyori | Functional abdominal pain |
Upper respiratory tract infections | |
Periodontal disease | |
Food allergy | |
Sudden infant death syndrome | |
Idiopathic thrombocytopenic purpura | |
Growth impairment |
Tests that detect H. pylori are divided into noninvasive and invasive tests. Invasive tests require endoscopy and gastric tissue biopsy for detecting the bacterium and include culture, rapid urease test, histopathology, polymerase chain reaction, and fluorescence in situ hybridization (FISH) tests [93]. On the other hand, noninvasive tests include different methods for the detection of H. pylori antigens in stool, detection of antibodies against H. pylori in different biological materials including serum, urine, and oral samples, and widely used 13C-urea breath test (13C-UBT) [93].
Noninvasive Tests
Among noninvasive diagnostic tests, stool antigen test and 13C-UBT have higher accuracy than serological or urinary antibody-based tests [94].
13C-Urea Breath Test
Recently published meta-analysis on the performance of the 13C-UBT showed good accuracy especially in children older than 6 years of age (sensitivity 96.6 %, specificity 97.7 %) [95]. A crucial question for all tests performed in a pediatric population is whether the accuracy of the applied method is influenced by the age of the tested child. 13C-UBT requires patient cooperation; moreover, patients need to fast before testing and drink without tracer withhold in the mouth. All that makes 13C-UBT difficult to perform in children younger than 6 years of age and may at least in part explain the lower specificity reported in young children [96, 97].
Stool Antigen Tests
Because H. pylori and its macromolecules such as proteins and DNA are shed in feces, stool-based tests have become advanced [98]. Several commercial enzyme-linked immunosorbent assay (ELISA) tests for H. pylori stool antigens are available. The main differences among these tests are the nature of the detecting antibodies; some kits use a polyclonal anti-H. pylori antibody, whereas other assays use monoclonal antibodies, and recently rapid one-step test (immunochromatographic format) using monoclonal antibodies has been introduced [98]. Meta-analysis on stool antigen-detection tests revealed that ELISA monoclonal antibodies have the best performance, with sensitivity and specificity of 97 % compared to ELISA polyclonal antibodies (sensitivity of 92 %, specificity of 93 %), and to one-step monoclonal antibody tests (sensitivity of 88 %, specificity of 93 %) [98]. So far, only the ELISA based on monoclonal antibodies has achieved the accuracy of the 13C-UBT, which is considered the reference standard of the noninvasive tests [4]. Use of stool tests is generally more convenient in pediatric patients than the 13C-UBT. It has several advantages: stool samples can be obtained from children without their active collaboration and are transportable by mail for analysis, the cost is lower comparing to 13C-UBT, and it is not age-dependent. Therefore, validation studies in adults may be extrapolated to children [4].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







