Gastrointestinal motility can be markedly deranged in critical illness. This can have a number of important clinical sequelae—the most obvious of which is impaired delivery of enteral nutrition, which can result in malnutrition, if not recognized and treated. Impaired gastric emptying and lower esophageal sphincter function may allow reflux of gastric contents into the esophagus during enteral feeding, especially in the recumbent position. This situation, combined with the loss of normal airway reflexes in the sedated and sometimes paralyzed patient, results in aspiration, which can be subclinical and therefore unrecognized, impairing respiratory function and predisposing to ventilator-associated pneumonia. Another proposed consequence of gastrointestinal dysmotility in the critically ill is intestinal stasis with bacterial overgrowth, potentially leading to bacterial translocation and nosocomial sepsis. However, this has never been conclusively demonstrated in humans.
Esophageal Motility
The gastroesophageal sphincter has reduced activity in critical illness, which can have important clinical consequences. Basal lower esophageal pressures are reduced when compared to health and acid reflux occurs frequently during fasting and gastric feeding. Furthermore, reflux contents remain in the esophagus for prolonged periods as clearance is markedly impaired. Most reflux episodes occur because of very low or, in some cases, absent lower esophageal sphincter pressures. Reflux episodes are also associated with straining and coughing on the endotracheal tube.
Gastric Motility
Gastric motility can be markedly abnormal in critical illness resulting in slow gastric emptying and reduced ability to tolerate nasogastric delivery of nutrients. The stomach may be functionally divided into proximal and distal parts and, in order to achieve optimal gastric emptying, the motility of these regions needs to be coordinated. During critical illness, not only is the motility of each region disturbed, but also the motor integration between the proximal and distal stomach is disrupted.
Fundal tone is important for normal gastric emptying of liquid nutrient and, is, therefore, likely to be fundamental to the gastric emptying of liquid formulae in the critically ill patient. In health, proximal gastric relaxation occurs in response to the presence of duodenal nutrient. In critical illness, accommodation of the proximal stomach in response to small intestinal nutrient is delayed and there is increased retention in the proximal stomach. These abnormal patterns of motility are likely to result in delayed distribution of ingesta to the distal stomach, which will slow gastric emptying and, potentially, increase the risk of gastroesophageal reflux.
Reduced fasting antral motility, which is characterized by the absence, or reduction, of the antral component of phase 3 of the interdigestive migrating motor complex (MMC), occurs in critical illness and is associated with slow gastric emptying. An absence or reduction of antral phase 3 activity may predispose to colonization of the stomach with microbial pathogens. In critically ill patients this could have serious consequences, as it may be a precursor of ventilator-associated pneumonia.
In health, the rate of gastric emptying is directly related to antral activity and, hence, delayed gastric emptying may be associated with weak and/or disordered antroduodenal contractions. Reduced postprandial antral activity has been reported in critically ill patients. Furthermore, erythromycin markedly increases antral wave activity and accelerates gastric emptying in this group. Thus, a direct relationship between gastric emptying and antral activity in the critically ill has been established.
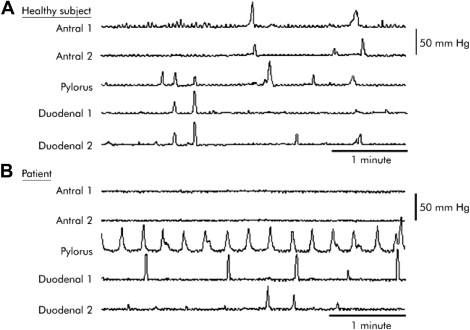
Pyloric activity (phasic and tonic) is integral to the regulation of gastric emptying. Both increased basal pyloric pressure and more frequent phasic contractions have been demonstrated in critically ill patients, and these abnormalities have been shown to correlate with slow gastric emptying ( Fig. 1 ).
Gastric Motility
Gastric motility can be markedly abnormal in critical illness resulting in slow gastric emptying and reduced ability to tolerate nasogastric delivery of nutrients. The stomach may be functionally divided into proximal and distal parts and, in order to achieve optimal gastric emptying, the motility of these regions needs to be coordinated. During critical illness, not only is the motility of each region disturbed, but also the motor integration between the proximal and distal stomach is disrupted.
Fundal tone is important for normal gastric emptying of liquid nutrient and, is, therefore, likely to be fundamental to the gastric emptying of liquid formulae in the critically ill patient. In health, proximal gastric relaxation occurs in response to the presence of duodenal nutrient. In critical illness, accommodation of the proximal stomach in response to small intestinal nutrient is delayed and there is increased retention in the proximal stomach. These abnormal patterns of motility are likely to result in delayed distribution of ingesta to the distal stomach, which will slow gastric emptying and, potentially, increase the risk of gastroesophageal reflux.
Reduced fasting antral motility, which is characterized by the absence, or reduction, of the antral component of phase 3 of the interdigestive migrating motor complex (MMC), occurs in critical illness and is associated with slow gastric emptying. An absence or reduction of antral phase 3 activity may predispose to colonization of the stomach with microbial pathogens. In critically ill patients this could have serious consequences, as it may be a precursor of ventilator-associated pneumonia.
In health, the rate of gastric emptying is directly related to antral activity and, hence, delayed gastric emptying may be associated with weak and/or disordered antroduodenal contractions. Reduced postprandial antral activity has been reported in critically ill patients. Furthermore, erythromycin markedly increases antral wave activity and accelerates gastric emptying in this group. Thus, a direct relationship between gastric emptying and antral activity in the critically ill has been established.
Pyloric activity (phasic and tonic) is integral to the regulation of gastric emptying. Both increased basal pyloric pressure and more frequent phasic contractions have been demonstrated in critically ill patients, and these abnormalities have been shown to correlate with slow gastric emptying ( Fig. 1 ).
Small Intestinal Motility
Slow gastric emptying will delay delivery of nutrient to the small intestine resulting in a reduced rate of nutrient absorption. Small intestinal motility is also impaired in critical illness, which has the potential to further reduce nutrient absorption and contribute to the malnutrition of critical illness. Abnormalities in MMC activity may be characterized by changes in the proportion of time spent in the 3 phases, the coordination of contractions during the phases, and/or the direction of migration of phase III activity. Furthermore, persistence of MMC activity during feeding is considered pathologic, although its implications are unclear. The relationship between the frequency of phase III activity and small intestinal transit is uncertain. Both more frequent MMC activity and delayed transit have been demonstrated following the administration of opioids and postoperatively. These are relevant to critical illness.
In critical illness, during fasting, the number of contractions and the occurrence of activity fronts in the duodenum (proximal and distal) are comparable to health. Although the duration of the duodenal MMC is also similar, the relative contribution of the quiescent period (phase 1) to the total cycle length is increased and that of phase 2 activity reduced. The activity fronts are also abnormal in character with retrograde or stationary propagation. Furthermore, fasting activity has been reported to persist in some critically ill patients during feeding, and, while the clinical implications of this are uncertain, data suggest that this may be associated with reduced absorption in patients following major abdominal surgery.
Small intestinal transit has rarely been evaluated in the critically ill, but it does not appear to be substantially faster than in health. This is relevant as rapid transit can attenuate absorption. In the critically ill, no relationship between transit and nutrient absorption has been demonstrated.
The normal intestinal microbiota stimulates the initiation and aboral migration of physiologic phase III activity and, conversely, loss of normal MMC activity is associated with small intestinal bacterial overgrowth in health. The microbiota in critical illness is unlikely to be normal but the effect of any abnormality on intestinal motility is unclear. While this relationship between motility and overgrowth has not been established in the critically ill, translocation of intestinal bacteria causing subsequent blood stream contamination is commonly considered to be a driver of multiorgan failure in this group. Thus, small intestinal dysmotility may have a role in the perpetuation of sepsis in critical illness. There are no data relating to motility of the large intestine in the critically ill.
Control of Gastrointestinal Motility
In health, nutrient interacts with small intestinal receptors and, via neurohumoral mechanisms, controls gastric emptying at a rate of about 2 to 3 kcal/min. This small intestinal feedback is exaggerated in the critically ill and appears to be the principal mechanism responsible for the abnormal slowing of gastric emptying during enteral feeding. The delivery of nutrient into the small intestine at a rate of as little as 1 kcal/min reduces antral and increases pyloric activity, which slows gastric empting; this same caloric load has no effect on gastric motility in health ( Fig. 2 ).

Antropyloroduodenal motility is mainly controlled by the enteric nervous system with modulation from extrinsic neurologic control and hormonal influences. Gastroparesis can, therefore, be caused by abnormalities in intrinsic or extrinsic neural innervation; however, the latter is more likely to be important. Vagal activity increases gastric emptying, so factors that interfere with vagal control, will be inhibitory. Vagal neuropathy is considered an important mediator of gastroparesis in ambulant diabetics and could be a factor in critical illness. The vagus is also important in the control of fasting motility. Abolition of fasting motility by food intake requires an intact vagus and, therefore, persistence of MMC activity during feeding suggests abnormal extrinsic neural control. The failure of abolition of fasting motility patterns by feeding in some critically ill patients may be a result of reduced vagal activity.
Sympathetic activity inhibits gastrointestinal motility and sympathetic overactivity is a feature of critical illness. Therefore, sympathetic neural stimulation may have a role in gastrointestinal dysmotility in the critically ill.
Numerous hormones are involved in the regulation of gastrointestinal motility ( Fig. 3 ). Cholecystokinin (CCK) is an important humoral mediator of the enterogastric feedback response in health, and also appears to have a role in feeding intolerance and delayed gastric emptying in the critically ill. Reduced fasting ghrelin concentrations and elevated fasting and postprandial peptide YY (PYY) levels have been measured in the critically ill ; however, the relative contributions of the different gastrointestinal hormones to the control of gastric emptying in the critically ill have not been studied.
The hormone that appears to be most important in the control of the MMC in health is motilin. Intravenous administration of motilin, or 1 to 3 mg/kg/h of the motilin agonist erythromycin, induces “phase III–like” activity in the antroduodenal region. Motilin levels peak during MMC activity. The effect of erythromycin on MMC activity is highly relevant to critically ill patients as erythromycin is used as a prokinetic. While fasting plasma motilin concentrations were recently shown to be similar in critically ill patients and healthy subjects, plasma motilin concentrations were significantly higher in the patients during nutrient infusion. In addition, there was an inverse relationship between the peak increase in plasma motilin concentrations and the peak change in proximal gastric volume induced by duodenal nutrient stimulation in critically ill subjects. These findings may explain the persistence in interdigestive gastrointestinal contractile activity and the impaired proximal gastric relaxation during enteral feeding in these patients.
Consequences of Gastrointestinal Dysmotility in the Intensive Care Unit
Gastroesophageal Reflux and Aspiration
Reflux episodes combined with the loss of normal airway reflexes in the sedated and sometimes paralyzed patient results in aspiration, which can lead to impaired respiratory function. The relationship between gastric dysmotility and reflux is, as yet, unclear but there is some evidence that feeding directly into the small intestine reduces the risk of reflux and nosocomial pneumonia.
Slow Gastric Emptying
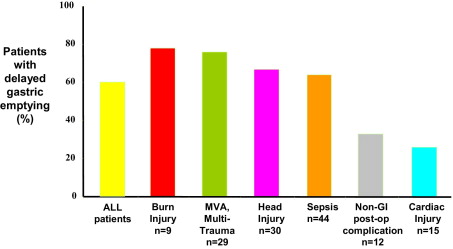
Gastric emptying is delayed in about 50% of mechanically ventilated, critically ill patients. Gastric emptying can be markedly slowed, with about 1 in 5 patients emptying less than 50% of gastric contents at 4 hours. Certain diagnostic groups appear more likely to have slow gastric emptying ( Fig. 4 ), and delays have been associated with increasing severity of illness. The cause of slow gastric emptying is likely to be multifactorial, with certain parameters having more or less importance in different patients. Additional factors associated with slow gastric emptying in the critically ill include raised intracranial pressure, reduced level of consciousness as measured using the Glasgow Coma Scale, time from traumatic brain injury, the height of a spinal cord lesion, increasing age, and administration of dopamine. Dopamine, a commonly used inotrope, reduces antral contractions, shortens MMC duration, and slows gastric emptying and orocecal transit time. This is likely to be mediated through central and peripheral effects. Other catecholamines, such as epinephrine, also stimulate beta-adrenergic receptors to slow gastric emptying. There are conflicting data in relation to the effects of the administration of opiates and gender. Electrolyte abnormalities such as hyperglycemia, recent surgery, shock, circulating cytokines, or the disease process itself are also likely to contribute. In health, acute elevations in blood glucose concentrations reduce gastroduodenal motility and slow gastric emptying, and high blood glucose concentrations occur frequently in the critically ill. We have demonstrated a relationship between hyperglycemia and feed intolerance ; however, this may not be causal as it is likely that factors which act to increase blood glucose concentrations, such as severity of illness and catecholamine requirement, may also slow gastric emptying. Interestingly, preexisting diabetes does not appear to be a risk factor for feed intolerance or delayed gastric emptying in critical illness.
Feed Intolerance
Intolerance to intragastric nutrition occurs commonly in critical illness. In a recent prospective, observational study in mechanically ventilated, intragastrically fed patients, the prevalence of “feed-intolerance” was approximately 35% (defined as a gastric residual volume of 250 ml or more), and the first episode of feed intolerance occurred early following admission to the ICU ( Fig. 5 ). However, the definition of feed intolerance is disputed and the implication of large gastric residual volumes (which are commonly used to define feed tolerance) is unclear. Volumes aspirated from the stomach are affected not only by the rate of gastric emptying but also by the rate of feed administration, gastric secretion, and duodenogastric reflux. The course of action that should be taken when a large gastric residual volume is obtained is debated. Currently, the majority of ICUs have protocols for feeding that recommend a change in delivery rate, or the initiation of a prokinetic drug or direct delivery of nutrient into the small intestine, if the gastric residual volume is between 150 and 500 ml. Our data suggest that a cumulative gastric residual volume over the preceding 24 hours of 150 ml or more has a positive predictive value of 0.78 (95% confidence interval [CI], 0.458 to 0.959) and a negative predictive value of 0.69 (95% CI, 0.423 to 0.893) for predicting slow gastric emptying, as subsequently measured by scintigraphy (the gold standard). However, the high rate of esophageal regurgitation and aspiration observed in these patients appears to be independent of gastric residual volume. As it is believed to be important to deliver energy goals to ICU patients, it appears unnecessary and inappropriate to change the rate of nutrient administration in response to large gastric volumes. However, as a large gastric residual volume suggests delayed gastric emptying, it may be logical to take action to improve nutrient delivery into the small intestine. This could be achieved by the administration of a prokinetic agent or the delivery of nutrient directly into the small intestine (see later).







