Fig. 10.1
Pathophysiologic mechanisms for GER. (Reproduced from Ref. [29], with permission from John Wiley and Sons)
GER occurs during episodes of TLESR or inadequate adaptation of the sphincter tone to changes in abdominal pressure. All the factors responsible for maintaining LES tone are not yet determined, but nitric oxide likely plays an important role. Infants have a short intra-abdominal esophagus.
Infants ingest more than twice the volume than adults per kilogram bodyweight (100–150 ml/kg/day compared to 30–50 ml/kg/day), causing more gastric distention and as a consequence more TLESRs. Feeding frequency is higher in infants than in adults, resulting in more postprandial periods during which TLESRs are more common. When investigated in supine position, the frequency of TLESRs in healthy adults and those with acid GERD does not differ. In healthy adults, only 30 % of the TLSERs are accompanied by acid reflux, but, in patients with GERD , reflux occurs in 65 % of the TLESRs. Thus, in adults, controls and GERD patients have the same number of TLESRs, but in patients with GERD, these TLESRs are more than twice as frequently accompanied with acid GER [30]. Older studies performed in adults in the recumbent position may be more relevant to understand the pathophysiologic mechanisms of acid reflux in infants. Normal individuals rarely experience TLESRs during sleep. Supine position removes all the beneficial gravitational effects of the erect position. Noxious materials, rather than air, are positioned at the cardia, available to move into the esophagus during TLESRs. A reflux is more likely to reach the pharynx in the recumbent than in the upright position. Both salivation and swallowing are markedly reduced during sleep, further impairing clearance. The upper esophageal sphincter is atonic during sleep, allowing reflux almost free to access the airways.
Delayed gastric emptying may increase postprandial reflux possibly by increasing the rate of TLESRs. Delayed gastric emptying has been documented in infants and children with symptomatic GER, particularly those with neurologic disorders. Abnormal gastric accommodation to a meal and prolonged postprandial fundic relaxation has been described in patients with GERD (M30 21). Esophageal acid exposure in patients with GERD is directly correlated with the emptying time of the proximal stomach. GERD was classically considered to be an acid peptic disease. But as a group, the majority of patients with reflux disease do not have a significant increase in gastric acid secretion. Recent analysis of postprandial acidity in the area of the gastroesophageal (GE) junction suggests that local acid distribution (“the gastric acid pocket”) rather than total gastric secretion might be more relevant to the pathogenesis of GERD. Differences may exist in the degree of mixing of fundic contents leading to different distributions of acid in the stomach. Studies using pH monitoring, scintigraphy and gastric magnetic resonance suggest that gastric mixing can be incomplete. Different layers of viscosity within the stomach might therefore influence the distribution of the gastric contents. A collection of acid in the gastric part of the esophageal junction was shown in adults in supine position, even in the postprandial period when stomach content was neutralized by the meal [31].
Hiatal hernia increases the number of reflux episodes and delays esophageal clearance by promoting retrograde flow across the esophagogastric junction when the LES relaxes after a swallow. This mechanism underlies the so-called re-reflux phenomenon (acid reflux when the pH is still below 4).
The majority of the studies on pathophysiologic mechanisms have been performed in adults and did not consider weakly acid and nonacid reflux. The refluxed material can be acid, weakly acid, or nonacid. Reflux may be a mix of gas and liquid or pure liquid, and it may or may not contain bile. More than half of the acid and weakly acid reflux episodes are associated with reflux of gas [30]. Weakly acid reflux also occurs predominantly during TLESRs. With liquid meals, patients with GERD had a similar total rate of reflux episodes but a higher proportion of acid reflux events than controls [32]. Weakly acid reflux may be responsible for the remaining symptoms in patients under antisecretory treatment. Components contributing to the noxiousness of refluxate are pepsin, bile acids and salts, and trypsin. The latter two depend on duodenogastric reflux preceding GER and are implicated in the genesis of strictures and Barrett’s esophagus. Acid is emptied from the esophagus with one or two sequences of primary peristalsis, and then the residual acidity is neutralized by swallowed saliva. Secondary peristalsis is the response to esophageal distension with air or water, and is more important during sleep when peristalsis is reduced. Patients may have normal primary peristalsis but abnormal secondary peristalsis. Thus, nonacid reflux that occurs in the postprandial period may be inefficiently cleared and cause prolonged esophageal distension, and thus cause symptoms of discomfort. Esophageal clearance modulates the duration of reflux episodes, while mucosal resistance modulates the noxiousness of the components of refluxate. Reflux may cause respiratory symptoms through different pathways, such as (micro-)aspiration or vagally mediated GER (Fig. 10.2).
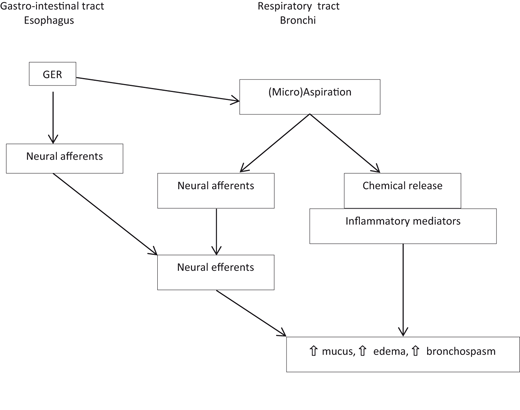

Fig. 10.2
Pathophysiologic mechanisms of GER causing respiratory disease. GER gastroesophageal reflux orig
Helicobacter pylori infection, or eradication of H. pylori, does not cause GER. Anyway, because of the decrease of H. pylori infection, this issue has become less relevant. Improvement in epigastric pain is significantly correlated with the improvement in GER symptoms but not with eradication of H. pylori [33].
The role of the upper esophageal sphincter in GERD and chronic respiratory disease, laryngitis, hoarseness, coughing, etc., has been insufficiently studied. The upper esophageal sphincter relaxes in response to esophageal body distention by gas, in contract to its contractile response to esophageal body distension by fluid. Symptom presentation has not been linked to different pathophysiologic mechanisms. Children presenting with upper airway disease or ear–nose–throat (ENT) manifestations may rather suffer from an insufficient upper esophageal sphincter, while patients with esophagitis may have more noxious reflux, insufficient clearing mechanisms or a poor esophageal mucosal resistance.
Symptoms and Signs
In normal 3–4-month-old infants, 3–4 episodes of GER are detectable during 5 min of intermittent fluoroscopic evaluation [34]. Normal ranges of esophageal pH monitoring report up to 31 ± 21 acid reflux episodes recorded within a 24-h period (but sample frequency, data handling by the recording device and program do determine this incidence) [35]. Attempts have been made to establish normal ranges in the pediatric age for esophageal impedance [36, 37]. However, for ethical reasons, these investigations were performed in symptomatic children.
While reflux does occur physiologically at all ages, there is also a continuum between physiologic GER and GERD at all ages leading to significant symptoms, signs, and complications (Tables 10.1 and 10.2). GERD is a spectrum of a disease that can best be defined as manifestations of esophageal or adjacent organ injury secondary to the reflux of gastric contents into the esophagus or, beyond, into the oral cavity or airways. The presenting symptoms of GERD differ according to age (Table 10.3). The list of most frequent differential diagnoses of vomiting in infants and children is listed in Table 10.4.
Table 10.1
Symptoms and signs that may be associated with gastroesophageal reflux. (Reproduced from Ref. [1], with permission from Lippincott Williams & Wilkins)
Symptoms | |
Recurrent regurgitation with/without vomiting | |
Weight loss or poor weight gain | |
Irritability in infants | |
Ruminative behavior | |
Heartburn or chest pain | |
Hematemesis | |
Dysphagia, odynophagia | |
Wheezing | |
Stridor | |
Cough | |
Hoarseness | |
Signs | |
Esophagitis | |
Esophageal stricture | |
Barrett’s esophagus | |
Laryngeal/pharyngeal inflammation | |
Recurrent pneumonia | |
Anemia | |
Dental erosion | |
Feeding refusal | |
Dystonic neck posturing (Sandifer syndrome) | |
Apnea spells | |
Apparent life-threatening events (ALTE) |
Table 10.2
Warning signals requiring investigation in infants with regurgitation or vomiting. (Reproduced from Ref. [1], with permission from Lippincott Williams & Wilkins)
Bilious vomiting |
GI bleeding |
Hematemesis |
Hematochezia |
Consistently forceful vomiting |
Onset of vomiting after 6 months of life |
Failure to thrive |
Diarrhea |
Constipation |
Fever |
Lethargy |
Hepatosplenomegaly |
Bulging fontanelle |
Macro/microcephaly |
Seizures |
Abdominal tenderness or distension |
Documented or suspected genetic/metabolic syndrome |
Table 10.3
Symptoms according to age. (Reproduced from Ref. [29], with permission from John Wiley and Sons)
Manifestations | Infants | Children | Adults |
|---|---|---|---|
Impaired quality of life | a | a | a |
Regurgitation | a | c | c |
Excessive crying/irritability | a | c | e |
Vomiting | b | b | c |
Food refusal/feeding disturbances/anorexia | b | c | c |
Persisting hiccups | b | c | c |
Failure to thrive | b | c | e |
Abnormal posturing/Sandifer’s syndrome | b | c | e |
Esophagitis | c | b | a |
Persistent cough/aspiration pneumonia | c | b | c |
Wheezing/laryngitis/ear problems | c | b | c |
Laryngomalacia/stridor/croup | c | b | e |
Sleeping disturbances | c | c | c |
Anemia/melena/hematemesis | c | c | c |
Apnea/ALTE/desaturation | c | e | e |
Bradycardia | c | f | f |
Heartburn/pyrosis | f | b | a |
Epigastric pain | f | c | b |
Chest pain | f | c | b |
Dysphagia | f | c | b |
Dental erosions/water brush | f | c | c |
Hoarseness/globus pharynges | f | c | c |
Chronic asthma/sinusitis | e | b | c |
Laryngostenosis/vocal nodules problems | e | c | c |
Stenosis | e | d | c |
Barrett’s esophageal adenocarcinoma | e | d | c |
Table 10.4
Differential diagnosis of vomiting in infants and children. (Reproduced from Ref. [1], with permission from Lippincott Williams & Wilkins)
GI obstruction | Pyloric stenosis |
Malrotation with intermittent volvulus | |
Intestinal duplication | |
Hirschsprung disease | |
Antral/duodenal web | |
Foreign body | |
Incarcerated hernia | |
Other GI disorders | Achalasia |
Gastroparesis | |
Peptic ulcer | |
Eosinophilic esophagitis/gastroenteritis | |
Food allergy | |
Inflammatory bowel disease | |
Pancreatitis | |
Appendicitis | |
Neurologic | Hydrocephaly |
Subdural hematoma | |
Intracranial hemorrhage | |
Intracranial mass | |
Infant migraine | |
Chiari malformation | |
Infectious | Gastroenteritis |
Sepsis | |
Meningitis | |
Urinary tract infection | |
Pneumonia | |
Otitis media | |
Hepatitis | |
Metabolic/endocrine | Galactosemia |
Hereditary fructose intolerance | |
Urea cycle defects | |
Amino and organic acidemias | |
Congenital adrenal hyperplasia | |
Renal | Obstructive uropathy |
Renal insufficiency | |
Toxic | Lead |
Iron | |
Vitamin A and D | |
Medications—ipecac, digoxin, theophylline, etc. | |
Cardiac | Congestive heart failure |
Vascular ring | |
Others | Pediatric falsification disorder (Munchausen syndrome by proxy) |
Child neglect or abuse | |
Self-induced vomiting (rumination syndrome) | |
Cyclic vomiting syndrome | |
Autonomic dysfunction |
Belching or eructation occurs during transient relaxation of the LES, and is an important method of venting air from the stomach. Hiccups are involuntary reflex contractions of the diaphragm followed by laryngeal closure. In some cases, hiccups cause GER.
Atypical symptoms such as epigastric pain, nausea , flatulence, hiccups, chronic cough, asthma, chest pain, and hoarseness account for 30–60 % of the presentations of GERD [1, 38] (Table 10.1). Possible associations exist between GERD and asthma, pneumonia, bronchiectasis, acute life-threatening event (ALTE), laryngotracheitis, sinusitis, and dental erosion, but causality or temporal association were not established [38]. The paucity of studies, small sample sizes, and varying disease definitions did not allow firm conclusions to be drawn [38]. Less than 10 % of infants and children have (acid and troublesome) GERD [35].
The clinician needs to be aware that not all regurgitation and vomiting in infants and young children is GER (disease). Bilious vomiting, gastrointestinal (GI) bleeding, consistently forceful vomiting, weight loss or failure to thrive, diarrhea, constipation , fever, lethargy, hepatosplenomegaly, and abdominal tenderness or distension should raise the possibility of an alternate diagnosis. Bulging fontanelle, macro- and/or microcephaly, and seizures raise the possibility of genetic and/or metabolic syndromes.
GER and Uncomplicated Regurgitation
Regurgitation is the most common presentation of infantile GER, with occasional projectile vomiting.
About 70 % of the healthy infants have regurgitation that is physiologic, resolving without intervention in 95 % of the individuals by 12–14 months of age [10–12] (Fig. 10.3). Daily regurgitation occurs more frequently in infants during the first 6 months of life than in older infants and children. Frequent regurgitation, defined as > three times per day, occurs in about 25 % of the infants during the first months of life. About 20–25 % of the parents seek medical advice because of frequent infantile regurgitation, which corresponds to at least four episodes of regurgitation a day [10–12].
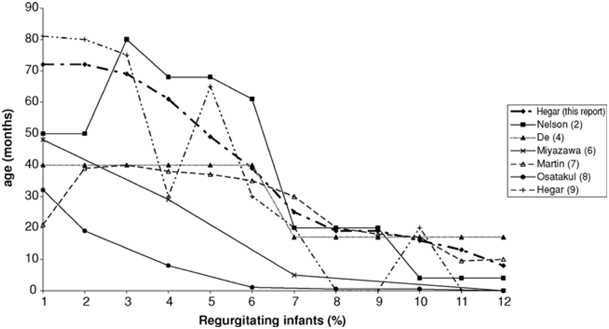

Fig. 10.3
Natural evolution of physiologic regurgitation. (Reproduced from Ref. [10], with permission from John Wiley and Sons)
A prospective follow-up reported disappearance of regurgitation in all subjects before 12 months, although an increased prevalence of feeding refusal, duration of meals, parental feeding-related distress, and impaired quality of life were observed, even after the disappearance of symptoms [12]. Regurgitation occurs more frequent in infants than in adults because of the large liquid volume intake, the limited capacity of the esophagus (10 ml in newborn infants), the horizontal position of infants, etc. “Excessive regurgitation” is one of the symptoms of GERD, but the terms regurgitation and GERD should not be used as synonyms.
Although most studies report a comparable incidence of regurgitation in unselected populations of formula versus breastfed infants, Hegar et al. reported a higher incidence in formula-fed infants [10]. This observation fits with the knowledge that GER and symptoms of GERD may be indistinguishable from those of food allergy [1, 2]. The incidence of cow’s milk protein allergy (CMPA) is five to ten times higher in formula fed than in breastfed infants [39] .
Regurgitation is a characteristic symptom of reflux in infants, but is neither necessary nor sufficient for a diagnosis of GERD, because regurgitation is neither sensitive nor specific. The physician’s challenge is to separate regurgitation and vomiting caused by reflux from numerous other disorders provoking the same manifestations. Although the “happy spitter” certainly exists (and is not rare), many infants show some symptoms of distress and discomfort when regurgitating. Irritability may accompany regurgitation and vomiting; however, in the absence of other warning symptoms, it is not an indication for extensive testing [1]. But in fact, parental carrying capacity or anxiousness (“parental coping”) will determine if a physician is contacted or not. Infant regurgitation is a benign condition with a good prognosis, needing no other intervention than parental education and anticipatory guidance, and intervention on feeding composition may contribute to parental reassurance. Overfeeding exacerbates recurrent regurgitation. Thickened or anti-regurgitation formula decreases overt regurgitation [1].
GER(D), Recurrent Regurgitation, and Poor Weight Gain
If poor weight gain is documented, it is obvious that the infant is not a happy spitter. Poor weight gain is a crucial warning sign that necessitates clinical management. These infants need a complete diagnostic workup, starting with a dietary history to evaluate caloric intake. Hospitalization of these infants may be needed. Although usually regurgitation causes little more than a nuisance, important regurgitation produces also caloric insufficiency and malnutrition in a minority of infants. There may be abnormal sucking and swallowing, and weight gain may be poor. These infants have no apparent malformations, and may be diagnosed as suffering “nonorganic failure to thrive,” a “disorder” that sometimes is attributed to social/sensory deprivation, socioeconomic or primary maternal–child problems. GERD is only one of the many etiologies of “feeding problems” in infancy. Poor weight gain, feeding refusal, back arching, irritability, and sleep disturbances have been reported to be related as well as unrelated to GERD [1, 2, 40].
GER(D) and Cow’s Milk Allergy
Guidelines on the symptoms and management of cow’s milk allergy unanimously report that persistent regurgitation and vomiting are manifestations of CMPA (Table 10.5: overlapping symptoms between GER(D) and CMPA) [41] . The positive response to cow’s milk protein elimination from the diet and relapse of the symptoms is often proposed as a proof of CMPA. An association between GERD and cow milk hypersensitivity was observed in both infants and children with severe GERD [43]. Simultaneous cow milk challenge and pH monitoring had limited value as a method to identify this subgroup. Impedance has shown that the incidence of nonacid postprandial reflux is decreased after a feeding with an amino acid-based formula compared to standard infants’ formula [44]. However, since amino acids or extensive hydrolysates have much more rapid gastric emptying than standard infants formula with entire cow milk proteins [45, 46], it is not possible to know if the decrease in GER is due to the enhanced gastric emptying or an immune mechanism .
Table 10.5
Symptoms attributed to GER and CMA. (Reproduced with permission from Ref. [42] by the AAP)
GER | GER + /−CMA | CMA |
|---|---|---|
Dysphagia | Crying | Diarrhea |
Hematemesis | Irritability | Bloody stools |
Melena | Colic | Rhinitis |
Rumination | Parental anxiety | Nasal congestion |
Nausea/belching | Feeding refusal | Anaphylaxis |
Arching | Failure to thrive | Constipation |
Bradycardia | Vomiting | Eczema/dermatitis |
Hiccups | Constipationa | Angioedema |
Sandifer’s syndrome | Regurgitation | Lip swelling |
Aspiration | Sideropenic anemia | Urticaria/itching |
Laryngitis/stridor | Wheezing | |
Respiratory infections | Apnea/ALTE/SIDS | |
Hoarseness | Sleep disturbances |
GERD and Esophagitis
Esophagitis is defined as visible breaks of the esophageal mucosa [1] . Histology is recommended to rule out complications (Barrett’s esophagus] or other causes of esophagitis (EoE). Reflux esophagitis is reported to occur in 2–5 % of the population. Children with GER symptoms present with esophagitis in 15–62 %, Barrett’s esophagus in 0.1–3 %, and refractory GERD requiring surgery in 6–13 % [1, 47]. Erosive esophagitis in 0–17-year-old children with GERD symptoms was reported to be 12.4 % and increasing with age 48. The median age of the group with erosive esophagitis was 12.7 + 4.9 years vs. 10.0 + 5.1 years in those without erosive esophagitis [48]. The incidence of erosive esophagitis was only 5.5 % in those younger than 1 year [48]. This finding is in sharp contrast with the extremely high incidence (24.8 %) of anti-reflux medication prescribed in extremely low-birth-weight infants at the moment of discharge [49]. The huge differences in incidence of esophagitis are determined by patient recruitment, differences of definition of esophagitis, and availability of self-treatment . Hiatal hernia is more frequent in children with erosive esophagitis than without (7.7 vs. 2.5 %) [48].
The primary symptom of an esophageal stricture is dysphagia. Barrett’s esophagus is not rare in adolescents with chronic GERD [1]. In adults, hospital discharges and mortality rates due to gastric cancer, gastric ulcer, and duodenal ulcer have declined during the past three decades, while those of esophageal adenocarcinoma and GERD have markedly risen [50] .
Esophagitis, identified by histology , occurs in 61–83 % of infants with reflux symptoms severe enough to perform endoscopy. Although esophagitis may present with pain, it can also be asymptomatic. The group with asymptomatic esophagitis is in some ways the most problematic. Even severe esophagitis may remain asymptomatic as demonstrated by children who present with peptic strictures without having experienced any discomfort attributable to esophagitis. Typical substernal burning pain (“heartburn,” pyrosis) occurs in many children suffering from esophagitis. Odynophagia, which is pain on swallowing, usually represents esophageal inflammation. In nonverbal infants, behaviors suggesting esophagitis include crying , irritability, sleep disturbance, and “colic” . Infants frequently also appear very hungry for the bottle until their first swallows and then become irritable, and refuse to drink. Dysphagia (“typical” for Eosinophilic esophagitis; EoE) has also been linked to esophagitis .
GER(D) and Eosinophilic Esophagitis
The impressive rise in prevalence of EoE is still poorly understood [51], and difficulty in distinguishing EoE from reflux esophagitis may be encountered . In reflux esophagitis, the distal and lower eosinophilic infiltrate is limited to less than 5/per high-power field (HPF) with 85 % positive response to GER treatment, compared to primary EoE with > 20 eosinophils per HPF. More recent, failure of proton pump inhibitor (PPI) treatment as a condition to diagnose EoE brought reflux esophagitis back in the picture of EoE [52]. EoE necessitates proper treatment (hypo-allergenic feeding, corticoids, montelukast, etc.). Patients with allergic esophagitis are younger and have atopic features (allergic symptoms or positive allergic tests), but have no specific symptoms. Atopic features are reported in more than 90 % and peripheral eosinophilia in 50 % of patients. At endoscopy, a pale, granular, furrowed, and occasional ringed esophageal mucosa may appear (M1 1). EoE is becoming more and more important [53]. While symptoms in older children are more oriented to dysphagia for solids, symptoms in infants are more reflux like [53, 54]. Repeated endoscopy with esophageal histology in combination with response to treatment may in some cases be the only way out to separate reflux esophagitis from EoE in young children. An in-depth discussion on EoE is included in Chap. 9.
GER(D), Heartburn, and Infant Crying
While the verbal child can communicate pain, descriptions of the intensity, location, and severity may be unreliable until the age of at least 8 years, and sometimes later [2]. In adults, adolescents, and older children, heartburn and regurgitation are the characteristic symptoms of GERD [7]. GERD in adolescents is more adult like. Heartburn is a symptom of GERD with or without esophagitis. Heartburn is a predominant GER symptom in adults, occurring weekly in 15–20 % and daily in 5–10 % of subjects. Diagnosis and management of GERD in older children ( > 12 years) and adolescents follows the recommendations for adults [1]. According to parents, heartburn is present in 1.8 % of 3–9-year-old healthy children and 3.5 % of 10–17-year-old adolescents; regurgitation is said to occur in 2.3 and 1.4 %, respectively, and 0.5 and 1.9 % need antiacid medication [12]. In self-reports, adolescents complain about heartburn in 5.2 % and regurgitation in up to 8.2 %, while antiacids are taken by 2.3 % and histamine receptor antagonists (H2RA) by 1.3 %, suggesting that symptoms of GER are not rare during childhood and are underreported by parents or overestimated by adolescents [12]. In infants, the issue is more complicated. As per definition “heartburn” suggests that the individual with heartburn feels a burning retrosternal pain, parents, and health-care providers almost automatically hypothesize that a “crying baby” or a “baby with colic” is likely to suffer from heartburn or “occult GER.” Therefore, acid-reducing medication is often prescribed in infants [49, 55]. Several randomized controlled trials were performed in this indication, and for once all results indicate the same conclusion: PPIs are useless to decrease crying and distressed behavior in newborns and infants [56–59]. Heine and coworkers showed the absence of any relation between crying duration and result of pH monitoring [15].
A symptom-based diagnosis of GERD in infants and young children remains difficult. The reason for the differences in presentation of GERD according to age remains unclear. The persistence of symptoms and progression to complications are unpredictable for a group of patients and for the individual patient. Overall, the correlation between symptoms, results of pH monitoring, acid perfusion test, and histology is poor.
GER(D) and Distressed Behavior
This group of patients is much more difficult to deal with than the infant with poor weight gain . The same amount of distress and crying may be evaluated by some parents as easily acceptable, while the same amount of crying will be unbearable for other parents. In fact, the degree of “coping” capacity of the parents decides if medical help is looked for. Many factors, such as tobacco smoke, may cause infant irritability. CMPA is another well-identified cause of infant irritability. There is substantial individual variability and some healthy infants may cry up to 6 h a day [1].
The concept that infant irritability and sleep disturbances are manifestations of GER is largely derived from adult data [1]. Although this hypothesis seems an acceptable extrapolation, we should be aware that there are not many data on this topic. GERD affects quality of life significantly in adults and probably also in children (and their parents); although, quality of life is more difficult to evaluate in infants and young children. The developing nervous system of infants exposed to acid seems susceptible to pain hypersensitivity despite the absence of tissue damage. In adults, NERD is a general accepted entity. Again in adults, impaired quality of life, notably regarding pain, mental health, and social function, has been demonstrated in patients with GERD, regardless of the presence of esophagitis [60]. In an unselected population, 28 % of the adults report heartburn, almost half of them weekly, with a significant impact on the quality of life in 76 %, especially if the symptoms are frequent and long lasting. Despite that, only half of the heartburn complainers seek medical help, although 60 % takes medications. Thus, some adults “learn to live with their symptoms,” and acquire tolerance to long-lasting symptoms, while others accept to live with an impaired quality of life. In infancy and young children, verbal expression of symptoms is often vague or impossible and persistent crying, irritability, back arching, feeding, and sleeping difficulties have been proposed as possible equivalents of adult heartburn. Infants with GERD learn to associate eating with discomfort and thus subsequently tend to avoid eating and develop an aversive behavior around feeds, although behavioral feeding difficulties are also common in control toddlers [61] . Esophageal pain and behaviors perceived by the caregiver (usually the mother) to represent pain (e.g., crying and retching) potentially affect the response of the infant to visceral stimuli and the ability to cope with these sensations, both painful and nonpainful. A placebo-controlled randomized trial with PPI in distressed infants showed an equal decrease in distressed behavior in the treatment and the placebo group [56]. To date, there is no evidence that acid-suppressive therapy is effective in infants who present solely with inconsolable crying. In infants and toddlers, there is no symptom or group of symptoms that can reliably diagnose GERD or predict treatment response. A pilot study suggested that a 40°supine position in a specially developed “Multicare Anti-Regurgitation (AR)-Bed” decreased regurgitation and infant irritability significantly [62].
GER(D), Dysphagia, Odynophagia, and Food Refusal
Dysphagia is the difficulty of swallowing; odynophagia is pain caused by swallowing. Although GERD is frequently mentioned as a cause of dysphagia or odynophagia, there are no pediatric data showing this relation. Dysphagia is a prominent symptom in patients with EoE . Feeding difficulty and/or refusal are often used to describe uncoordinated sucking and swallowing, gagging, vomiting, and irritability during feeding. A relation between GER, GERD, and feeding refusal has not been established. In case of feeding difficulties, achalasia and foreign body should be among the list of possible differential diagnoses.
GER(D) and Extraesophageal Manifestations
Although evidence is sufficient to support an association between extraesophageal symptoms and GER, establishing that an individual patient’s extraesophageal symptoms are caused by reflux is difficult . Pulmonary microaspiration as demonstrated by pepsin detection in bronchoalveolar liquid (BAL) fluid is common in children with chronic lung diseases, suggesting that GER may contribute significantly to the disease pathogenesis [63]. BAL pepsin concentration correlates positively with the number of proximal reflux events [63]. Protein oxidation in BAL is higher in children with extensive proximal acidic reflux, suggesting that pulmonary microaspirations contribute to lung damage [63] .
GER(D) and Reactive Airway Disease
An etiologic role for GER in reactive airway disease has not been demonstrated . Different pathophysiologic mechanisms are proposed: direct aspiration, vagal-mediated bronchial and laryngeal spasm, and neural-mediated inflammation. Esophageal acidification in infants with wheezing can produce airway hyperresponsiveness and airflow obstruction [64]. Few studies tempted to evaluate the opposite: the impact of asthma on the severity of GERD. Chronic hyperinflation that occurs in asthma favors many GER mechanisms. An association between asthma and reflux measured by pH or impedance probe has been reported in many studies (M7, M37). Wheezing appears more related to GERD if it is nocturnal. A recent study reports a high prevalence of GER in children and adolescents with persistent asthma, equally distributed in the supine (nocturnal) and upright positions [65]. But, there was no correlation between the result of the pH-metry and pulmonary function tests [65]. There are no studies that help in selecting patients in whom reflux treatment may result in a reduction of asthma medication, if there are such patients at all (M1, M37) .
Very few prospective, randomized, and blinded treatment studies have been performed in children. In a series of 46 children with persistent moderate asthma despite bronchodilators, inhaled corticosteroids, and leukotriene antagonists, 59 % (27/46) had an abnormal pH-metry [66]. Reflux treatment did result in a significant reduction in asthma medication. Patients with a normal pH-metry were randomized to placebo or reflux treatment: 25 % (two of only eight children) of the treated patients could reduce their asthma medication, while this was not possible in any patient on placebo [66]. Another study found omeprazole ineffective in improving asthma symptoms and parameters in children with asthma [67]. Overall, although there seems to be an association between GER and asthma, the causal role of GER has not been demonstrated. There is no association between asthma control status and laryngo-pharyngeal reflux and GER [68]. Current evidence does not support the routine use of anti-GERD medication in the treatment of poorly controlled asthma of childhood [69] .
GER(D) and Recurrent Pneumonia
The reported mechanisms are similar to those for reactive airway disease. Direct aspiration during swallowing may be more relevant in this group . No test can determine whether reflux is causing recurrent pneumonia. Upper esophageal and pharyngeal pH and impedance recordings provided contradictory information. Today, it is not yet clear if recording in the upper esophagus or pharynx will help in making therapeutic decisions [70, 71]. A new technique to record pharyngeal reflux has been developed (Restech®) [71]. However, recent results could not confirm that the technique is reliable if compared to esophageal impedance [72].
Lipid-laden macrophages have been used as an indicator of aspiration, but their sensitivity and specificity for GER is poor. One study evaluating nuclear scintigraphy with late imaging reported that 50 % of the patients with a variety of respiratory symptoms had pulmonary aspiration after 24 h [73]. However, later studies failed to reproduce these findings [74]. Aspiration also occurs in healthy subjects, especially during sleep [1].
The role of reflux in patients with bronchopulmonary dysplasia and other chronic respiratory disorders is not clear. Today, the clinician has frequently no other option than to make management decisions based on inconclusive diagnostic studies with no certainty regarding outcome [1]. As in reactive airway disease, it is very likely (although not evidence based proven) that (not all) reflux needs to be acid to cause airway manifestations. However, today, medical treatment options are limited to acid-reducing medication .
GER(D) and Cystic Fibrosis
The role of GER in adults and children with cystic fibrosis (CF) has been studied before and after transplant [75, 76]. Acid reflux exists in the majority of CF patients [1] . A high prevalence of acid GER was reported in very young CF infants even before respiratory symptoms developed [75]. CF patients also suffer from duodeno-gastroesophageal reflux of bile acids [77]. It is possible that the acid and bile reflux are aggravating the respiratory symptoms, and that the respiratory symptoms aggravate the reflux. Aggressive medical and surgical reflux treatment in this patient group seems reasonable. In children with CF , a better weight gain was reported during PPI treatment (whether this is due a reduction of acid reflux or better buffering of acid gastric content in the intestine is not clear) .
GER(D) and ENT Manifestations
Both acid and weakly acid GER may precede cough in children with unexplained cough, but cough does not induce GER [78] . Objective cough recording improves symptom association analysis (M75 ZCC]. Several studies revealed the presence of pepsin in the middle-ear fluid, but with a huge variation in incidence (14–73 %) [1, 79]. Also bile acids have been detected in middle-ear liquid, in higher concentrations than in serum [80]. The presence of pepsin and bile in middle ear fluid might as well be the consequence of reflux (and vomiting) at the moment of the acute middle ear infection than an argument to hypothesize that chronic GER may be at the origin of the chronic middle ear problem. However, several epidemiologic studies suggest a low incidence of reflux symptoms in patients with recurrent middle ear infections.
Data suggesting a causal relation between reflux and upper airway disease in children are limited. Data from several placebo-controlled studies and meta-analyses uniformly have shown no effect of anti-reflux therapy on upper airway symptoms or signs [1]. Well designed, prospective, placebo-controlled, blinded studies are needed. Another bias might be the selection of patients: These studies are frequently setup in tertiary care centers in highly selected patient populations. The question is how representative these patients are for the bulk of children with upper respiratory and/or ENT manifestations .
GER(D) and Dental Erosions
Young children and children with neurologic impairment appear to be at greatest risk to have dental erosions caused by GER . Juice drinking, bulimia, and racial and genetic factors that affect dental enamel and saliva might be confounding variables that have been insufficiently considered [1]. Recently, a positive correlation between GERD and dental erosion has been as well confirmed as refuted [81, 82]. There are no long-term (intervention) follow-up studies in high-risk populations.
GER(D) and Sandifer Syndrome
Sandifer syndrome (spasmodic torsional dystonia with arching of the back and opisthotonic posturing, mainly involving the neck and back) is an uncommon but specific manifestation of GERD .
GER(D) and Neurologic Impairment
Children with neurologic impairment have more frequent, more severe, and more difficult to treat GERD than neurologically normal children . Neurologically impaired children accumulate many risk factors for severe GERD: spasticity or hypotonicity, supine position, constipation, etc. Diagnosis of reflux disease in these children is often difficult because of their underlying conditions. Whether this group of patients has more severe reflux disease, or has less effective defense mechanisms, or presents with more severe symptoms because of the inability to express and/or recognize symptoms remains open for debate. Response to treatment, both medical and surgical, is poor in the neurologically impaired child compared to the neurologically normal child .
GER(D) and Apnea, ALTE and SIDS
Literature can best be summarized as follows: series fail most of the time to show a temporal association between GER and pathologic apnea, ALTE, and bradycardia [1, 83] . However, a relation between GER and short, physiologic apnea has been shown [84]. GER is a frequent cause of interrupting sleep in infants, and nonacid GER is equally important as acid GER for causing arousals and awakenings in infants [85]. Discomfort is significantly associated with reflux events and does not differ between weakly acidic and acid refluxes [86]. There are well-selected cases or small series that demonstrate that pathologic apnea can occur as a consequence of GER. However, in general, GER is not related to pathologic apnea, significant bradycardia, ALTE, and sudden infant death syndrome (SIDS) [83] .
GER(D) and Other Risk Groups
Symptomatic GER is extremely frequent in patients treated for esophageal atresia and/or tracheoesophageal fistula because of serious structural and functional deficiencies [87] . It is refractory to medical treatment and often requires anti-reflux surgery. However, the high rates of wrap failure invite close follow-up in all cases and reoperation or other measures whenever necessary [87]. Children with congenital abnormalities or after major thoracic or abdominal surgery are at risk for developing severe GERD. Children with anatomic abnormalities such as hiatal hernia, repaired esophageal atresia and malrotation have frequently severe GERD. There are no data in the literature that preterm babies have more (severe) reflux than term-born babies, although many preterms are treated for reflux .
GERD and Complications
Barrett’s esophagus, strictures, and esophageal adenocarcinoma are complications of chronic severe GERD . Barrett’s esophagus is a premalignant condition in which metaplastic specialized columnar epithelium with goblet cells is present in the tubular esophagus. Differences in esophageal mucosal resistance and genetic factors may partially explain the diversity of lesions and symptoms .
More than 50 years ago, in the absence of reflux treatment, esophageal strictures were reported in about 5 % of the children with reflux symptoms [88]. Currently, esophageal stenosis and ulceration in children have become rare. In a series including 402 children with GERD without neurological or congenital anomalies, no case of Barrett’s esophagus was detected [47]. In another series including 103 children with long-lasting GERD, and not previously treated with a H2RA or a PPIs , Barrett’s esophagus was detected in 13 %. An esophageal stricture was present in 5 of the 13 patients with Barrett’s esophagus (38 %) [89]. Reflux symptoms during childhood were not different in adults without than in adults with Barrett’s esophagus[90]. Barrett’s esophagus has a male predominance and increases with age. Patients with short segments of columnar-lined esophagus and intestinal metaplasia have similar esophageal acid exposure but significantly higher frequency of abnormal bilirubin exposure and longer median duration of reflux symptoms than patients without intestinal metaplasia [91]. There is a genetic predisposition in families in patients with Barrett’s esophagus and esophageal carcinoma [1] .
Children with neurological impairment , chronic lung disease (especially CF), esophageal atresia, and chemotherapy have the most severe pathologic reflux and are at high risk for the development of complications of GERD [1].
Peptic ulcer, esophageal and gastric neoplastic changes in children are extremely seldom. In adults, over the past 30 years, a decreased prevalence of gastric cancer and peptic ulcer with an opposite increase of esophageal adenocarcinoma and GERD has been noted. This has been attributed to independent factors among which changes in dietary habits such as a higher fat intake, an increased incidence of obesity and a decreased incidence of H. pylori infection. Recent data suggest obesity does not play a major role [92] The incidence of noninvasive in situ cancer has actually declined after 2003 [93]. Frequency, severity, and duration of reflux symptoms are related to the risk to develop esophageal cancer. Among adults with long-standing and severe reflux, the odds ratios are 43.5 for esophageal adenocarcinoma and 4.4 for adenocarcinoma at the cardia [94]. It is unknown whether mild esophagitis or GER symptoms persisting from childhood is related to an increased risk for severe complications in adults. However, Barrett’s esophageal adenocarcinoma affects young individuals [95] .
Diagnosis
Diagnostic procedures are not discussed in full detail. Detailed information regarding indications and pitfalls of radiologic contrast studies, nuclear reflux scintigraphy, ultrasound, pH-metry , intraluminal impedance, endoscopy, manometry, gastric emptying tests, and electrogastrography can be found in previous review papers and guidelines [1].
In adults, diagnosis of GER disease is mainly based on clinical history. However, history in children is difficult and considered poorly reliable up to the age of minimally 8 or even 12 years old. Therefore, questionnaires have been developed trying to improve history reliability. Orenstein developed the “infant GER questionnaire” [96]. The questionnaire results in an objective, validated, and repeatable quantification of symptoms suggestions GERD. The I-GER was revised (the “I-GERQ-R”) in 185 patients and 93 controls, resulting in an internal consistency reliability from 0.86 to 0.87, and test-retest reliability was 0.85 [97]. However, Aggarwal and coworkers showed that the I-GER-Q had a sensitivity of only 43 % and a specificity of 79 % [98]. Moreover, pH-metry results were not different according to a “positive” or “negative” score of the I-GER-Q [98]. Our group showed that not one question was found to be significantly predictive for the presence of esophagitis. In our hands, the Orenstein I-GERQ cut-off score failed to identify 26 % of the infants with GERD (according to pH-metry results or presence of esophagitis), but was positive in 81 % of the infants with a normal histology of esophageal biopsies and normal pH-metry [99]. Deal et al. developed two different questionnaires, one for infants and one for older children, and showed that the score was higher in symptomatic than in asymptomatic children [100]. In other words, the correlation between the results of history obtained by questionnaires and of reflux investigations is poor .
Barium contrast radiography, nuclear scintiscanning, and ultrasound are techniques evaluating postprandial reflux and provide limited information on gastric emptying. Normal ranges are not established for any of these procedures. Barium studies are not recommended as first-line investigation to diagnose GERD, but are of importance to diagnose anatomic abnormalities such as malrotation, duodenal web, stenosis, and may suggest functional abnormalities such as achalasia , etc. Nuclear scintigraphy may show pulmonary aspiration [73]. Also, aspiration of saliva and gastric contents occurs during sleep in healthy adults [101]. Scintigraphy also can estimate gastric emptying. But the 13C-octanoic acid (for solids) and 13C-acetate (for liquids) breath tests are more appropriate to measure gastric emptying. The role of delayed gastric emptying in GER(D) remains controversial. Ultrasound provides morphological and functional data with high sensitivity and positive predictive value for the diagnosis of GER [102]. Sonographic assessment of findings such as abdominal esophageal length, esophageal diameter, esophageal wall thickness, and gastroesophageal angle provide important diagnostic indicators of reflux and related to the degree of GER [102]. However, there is a need for standardization of the procedure and for defining diagnostic criteria [102]. The results of ultrasound are investigator dependent, and a relation between reflux seen on ultrasound and symptoms has not been established [1]. There is no indication for electrogastrography in the diagnostic work up of a patient suspected of GERD.
Modern endoscopes are so much miniaturized that endoscopy of preterm infants of less than 1000 g has become technically easy. Operator experience is an important component of interobserver reliability [1]. Endoscopy allows direct visual examination of the esophageal mucosa. Macroscopic lesions associated with GERD include esophagitis, erosions, exudate, ulcers, structures, hiatal hernia, etc. Redness of the distal esophagus in young infants is a normal observation because of the increased number of small blood vessels at the cardiac region. Endoscopy may also show a “sliding hernia,” the stomach that is protruding in the esophagus during burping. Recent consensus guidelines define reflux esophagitis as the presence of endoscopically visible breaks in the esophageal mucosa at or immediately above the GE junction [1, 2]. Endoscopy-negative reflux disease is common. There is a poor correlation between the severity of symptoms and presence and absence of esophagitis. There is insufficient evidence to support the use of histology to diagnose or exclude GERD. Biopsies of duodenal, gastric, and esophageal mucosa are mandatory to exclude other diseases [1]. More detailed information on pros and cons of histology can be found in the recent consensus papers [1, 2] .
Intraluminal esophageal acid perfusion provoking chest pain (Bernstein test) or using other endpoints has found expanded use in practice and research in the USA, but was never popular in Europe. Ambulatory 24-h esophageal pH monitoring measures the incidence and duration of acid reflux, while impedance measures all reflux episodes. Esophageal pH-metry is the best method to measure acid in the esophagus, but not all reflux-causing symptoms are acid and not all acid reflux are causing symptoms. Esophageal pH-metry is useful in evaluating the effect of a therapeutic intervention on reducing esophageal acid exposure. Medical treatment is nowadays focusing on the reduction of gastric acid secretion; the technique offers the possibility to measure intragastric and esophageal recording of pH simultaneously. Both hardware (electrodes, devices) and software influence the results [1]. Normal ranges have been established for pH-metry. However, normal ranges depend also on the hard- and software used and are of limited value for reflux-causing extraesophageal manifestations. It becomes more and more obvious that the major indication of long-term recording of pH and/or impedance is the demonstration of an association between reflux and symptoms.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







