- Gastric cancer is the fourth most common malignancy in the world and is diagnosed at an advanced stage in more than 50% cases in the West.
- Upper GI endoscopy with biopsy remains the gold standard in diagnosis.
- Five-year overall survival is halved for every stage progression, with 5-year survival of 50–60% in stage I and 6–8% in stage IV.
- The main risk factors for gastric cancer are Helicobacter pylori infection, cigarette smoking, chronic atrophic gastritis, gastric ulcers, prior partial gastrectomy, and gastroesophageal reflux disease.
- Standard treatment approaches for resectable gastric and gastroesophageal junction tumors are perioperative chemotherapy, adjuvant S1 in East Asia, and postoperative chemoradiotherapy in North America.
- Palliative combination chemotherapy improves survival and quality of life and is indicated for patients with metastatic disease, with the addition of trastuzumab in patients with HER-2 overexpressing tumors.
UK cancer information, funding and research
British Society of Gastroenterology—education, training, research resources, and patient information
UK patient support and information site
European Society of Medical Oncology—updated clinical practice guidelines, educational manuscripts, and fellowship opportunities
US National Comprehensive Cancer Network—clinical practice guidelines in oncology
American Society of Clinical Oncology—education, public policy, and research resources
Patient support and information site supported by the ASCO Cancer Foundation
- Treatment of gastric cancer requires multidisciplinary input from pathology, gastroenterology, radiology, surgery, oncology, as well as allied health professionals.
- Adjuvant treatment should be given after gastrectomy to patients who did not receive any neoadjuvant treatment.
- Patients with metastatic disease in good performance status should be referred for palliative chemotherapy.
- Specialists in palliative medicine should be involved with patients failing chemotherapy.
Epidemiology
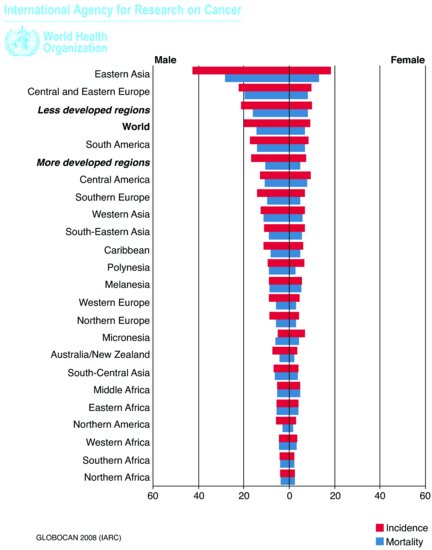
Gastric adenocarcinoma is the most frequent histological type of gastric malignancy (90%); other types include non-Hodgkin’s lymphomas (Chapter 10), leiomyosarcomas, gastrointestinal (GI) stromal tumors, and neuroendocrine tumors (Chapter 9). Gastric cancer remains the second leading cause of cancer death worldwide: the estimated incidence is 1 million new cases a year, making it the fourth most common malignancy in the world, after lung, breast, and colorectal cancers.1 Incidence rates are twice as high in men as in women, with the highest rates occurring in Eastern Asia and Eastern Europe and the lowest rate in Northern and Western Africa (Figure 3.1). In the European Union, there were approximately 86,000 new cases of gastric cancer diagnosed in 2006. Regional differences have been attributed to environmental factors, such as high dietary intake of salt, smoked, and cured meats; in addition, there are significant differences in incidence across socioeconomic groups. Gastric cancer incidence increases with age and rises rapidly from the age of 60 years onward. Although the age-standardized incidence of gastric cancer in the United Kingdom has been steadily decreasing since 1975, the incidence of tumors of gastroesophageal junction (GEJ) is rising.
Figure 3.1 Estimated age-standardized rates per 100,000. (Reproduced from Ferlay, J., Shin, H.R., Bray, F., Forman, D., Mathers, C. and Parkin, D.M.: GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France, International Agency for Research on Cancer, 2010, http://globocan.iarc.fr)1
Diagnosis
Patients with early gastric cancers can be relatively asymptomatic and are often picked up incidentally with iron-deficiency anemia. Locally advanced disease can present with anorexia, early satiety, abdominal discomfort, or pain. Tumors involving the gastric cardia can cause dysphagia, whereas distal tumors involving the pylorus can manifest with nausea and vomiting caused by gastric-outlet obstruction. The most common sites of distant metastases are the liver, lungs, and bone; symptoms that may suggest distant spread include weight loss, shortness of breath, and bone pain. All advanced cancers, especially involving the upper GI tract, can cause weight loss. Melena and hematemesis are usually signs of bleeding locally advanced cancer.
Physical examination in the early stages is often unrevealing. In more advanced stages of disease, patients may have an upper abdominal mass, palpable peritoneal deposits, or hepatomegaly secondary to liver metastases. A classical sign of metastatic gastric cancer is the Virchow’s node, a palpable left supraclavicular lymph node that develops when thoracic duct is blocked by metastases from abdominal cancers. Signet-ring, mucin-producing diffuse gastric cancer often metastasizes to the ovaries, a finding called Krukenberg tumor. A palpable metastatic nodule in the umbilicus known as Sister Mary Joseph nodule is thought to arise by lymphatic spread along the hepatoduodenal and falciform ligaments. Blood tests reveal iron-deficiency anemia in more than 50% of patients and patients with liver metastases may have abnormal liver function tests. Tumor markers including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (Ca19-9) have been found to have a low sensitivity and specificity for the detection of gastric cancer but can be used for monitoring of response to treatment.2
Guidelines for urgent referral for upper GI endoscopy have been published in an attempt to improve early diagnosis and are summarized in Table 3.1.3 However, patients with a high clinical suspicion including those 55 years and older with unexplained, persistent recent onset of dyspepsia who do not fulfill guideline criteria should still be urgently referred for upper GI endoscopy. Direct visualization of tumor with endoscopy and biopsy remains the gold standard for diagnosis of gastric cancer. In addition to upper GI endoscopy with biopsies, double-barium GI series can be helpful in defining gastric distensibility and obliteration of the gastric folds, especially in linitis plastica tumors.
Table 3.1 National Institute for Health and Clinical Excellence (NICE) guidelines for referral for urgent upper GI endoscopy.3
| Patients of any age with dyspepsia presenting with |
| Chronic gastrointestinal bleeding |
| Progressive unintentional weight loss |
| Progressive difficulty swallowing |
| Persistent vomiting |
| Iron-deficiency anemia |
| Epigastric mass |
| Suspicious barium meal |
The UICC 2009 TNM classification (7th edition) clinically and pathologically stages primary gastric cancers into four T stages (based on depth of invasion), three N stages (with minimum of 16 lymph nodes recovered to accurately assign pN stage), and M stage (the presence of metastatic disease), which is usually based on the clinical or radiological findings. The regional lymph node of the stomach includes perigastric (along lesser and greater curvature), the nodes along the left gastric, common hepatic, splenic and coeliac arteries, and hepatoduodenal nodes. Involvement of all other lymph nodes is classified as distant metastasis (M1 stage) (Table 3.2).4
Table 3.2 UICC TNM classification of gastric cancer (7th edition).4

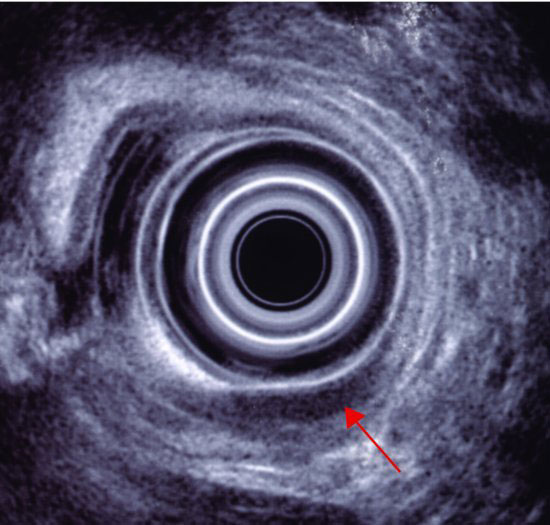
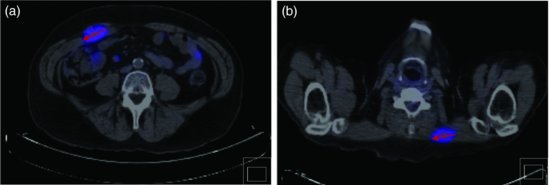
Computerized tomography (CT) of the chest and abdomen is used for staging gastric cancers and although it can be difficult to accurately assess the thickness of gastric cancer (T stage) on the CT due to variability in gastric filling, CT scans provide important information regarding the lymph node (N stage) and distant organ (M stage) involvement. CT scanning can determine lymph nodes greater than 5 mm; however, its sensitivity for tumor involvement is only 40–50% (Figures 3.2 and 3.3). Endoscopic ultrasound (EUS) is being used more frequently to complement upper GI endoscopy and CT scans in the staging of gastric cancer (Figure 3.4). EUS can accurately assess the invasion of any of the five stomach layers and define distal and proximal extent of the tumor. This is especially useful in assessing early tumors and tumors arising at the GEJ. Studies demonstrate 92% accuracy of EUS in T staging compared with 42% for CT.5 Nevertheless, the use of EUS for N staging is suboptimal due to low penetration of transducer and lymph node visualization. Although 18-fluorodeoxyglucose positron emission tomography (18FDG-PET) scans have revolutionized imaging in oncology in the last decade, only 41–83% of gastric cancers are 18FDG avid. Mucinous and signet-ring cells tumors have less prominent 18FDG uptake; tumors at the GEJ tend to be more avid and 18FDG-PET positive.6 18FDG-PET scans remain a valuable tool complementing CT in detection of distant metastases (Figures 3.5 and 3.6).7 There is evidence to suggest that 18FDG-PET is a useful predictor of early response to neoadjuvant chemotherapy and a strong prognostic indicator; in a trial of 35 patients with 18FDG avid tumors, the 2-year survival rate in metabolic responders after 2 weeks of neoadjuvant chemotherapy was 90% compared with 25% in the patients who did not achieve a metabolic response.8 A staging laparoscopy should be performed in all patients being considered for radical resection as staging CT scans can underestimate the extent of disease in up to 37% of patients.9 Laparoscopy allows direct visualization of peritoneal cavity and peritoneal cytology can be performed. The detection of malignant cells in peritoneal washings identifies patients at very high risk of recurrence and is associated with poor prognosis.10 Other histological factors that predict for worse prognosis include poorly differentiated tumors, lymphovascular invasion, and tumors with an infiltrative, diffuse growth pattern.
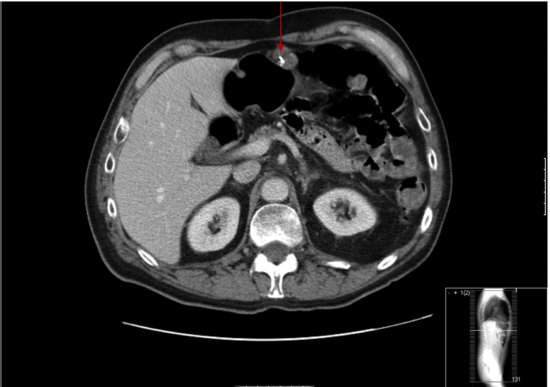
Figure 3.2 Primary tumor is not easily visualized on this CT scan and is confined to the wall of the stomach (Case 1).
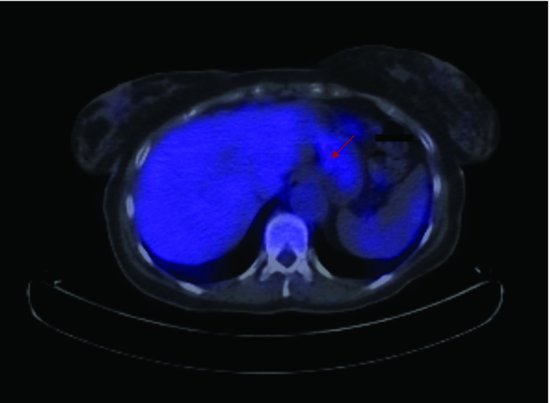
Figure 3.6 CT/PET scan demonstrating avid 18FDG uptake in the rectus abdominis (a) and trapezius (b) muscle (Case 2).
Prevention
The incidence of gastric cancer in the offspring of immigrants from countries with high incidence falls to that of the new home country, suggesting that development of gastric cancer depends on environmental exposure, with external and dietary carcinogens being the most likely factors. The main identified risk factors for gastric cancer are Helicobacter pylori (H. pylori) infection, various dietary and occupational exposures, medical conditions, and family history (Table 3.3). Observational cohort studies and meta-analysis suggest that aspirin use might reduce risk of adenocarcinoma of stomach, particularly distal, intestinal type11–13; however, further research on the potential of chemoprevention with aspirin or other nonsteroidal anti-inflammatory drugs is needed in high-risk populations.
Table 3.3 Risk factors for development of gastric cancer.
| Infection | Helicobacter pylori |
| Environmental | Dietary factors |
| Cigarette smoking | |
| Medical | Chronic atrophic gastritis |
| Gastric ulcers | |
| Prior partial gastrectomy | |
| GERD | |
| Pernicious anemia | |
| Gastric atrophy | |
| Hereditary | HDGC |
| Li–Fraumeni syndrome | |
| HNPCC | |
| Cowden’s syndrome | |
| Peutz–Jeghers syndrome | |
| GERD, gastroesophageal reflux disease; HDGC, hereditary diffuse gastric cancer; HNPCC, hereditary nonpolyposis colorectal cancer. | |
H. pylori is defined as gastric carcinogen by WHO and is the most important known risk factor for noncardiac gastric cancer14; infection with H. pylori doubles the risk of gastric cancer. Early H. pylori eradication in patients with peptic ulcer disease lowers the incidence of gastric cancer to that of the general population.15 Interestingly, there is a suggestion that H. pylori may have a protective effect in the development of GEJ adenocarcinomas.
A typically Mediterranean diet consisting of high dietary intake of olive oil, unrefined cereals, fruit and vegetables—with moderate consumption of cheese, yogurts, fish, and wine—and low intake of meat not only provides protection against heart disease and diabetes but also reduces incidence of stomach cancer by 33%.16 An active program of gastric cancer prevention is justified only in countries with high incidence, such as in East Asia. Nationwide screening of gastric cancer has been conducted since 1983 in Japan and has resulted in earlier diagnosis and declining mortality rates.17 Outside Japan, mass screening has not proven to be cost-effective.
Hereditary diffuse gastric cancer (HDGC) is a genetic syndrome characterized by a high risk of stomach and breast cancers. Gastric cancers occurring in this syndrome are typically diffuse, infiltrating the entire stomach (linitis plastica) and tend to have a signet-ring appearance. Hereditary gastric cancer is inherited in an autosomal dominant pattern and a germline mutation of the E-cadherin gene (CDH1), a tumor suppressor gene, has been identified in 30–50% of these families. Affected individuals have a lifetime risk up to 80% of developing gastric cancer. The average age for diagnosis of gastric cancer in these individuals is 38 years and affected individuals with a CDH1 mutation should be counseled regarding a prophylactic gastrectomy. Table 3.4 lists the criteria for referral for CDH1 testing.18
Table 3.4 Updated consensus guidelines on HDGC, International Gastric Cancer Linkage Consortium, Cambridge 2008.18
| 1. Two or more cases of gastric cancer in a family, with at least one diffuse gastric cancer diagnosed before the age of 50 years |
| 2. Three or more cases of diffuse gastric cancer in first- or second-degree relatives diagnosed at any age |
| 3. An individual diagnosed with diffuse gastric cancer before 40 years of age |
| 4. An individual diagnosed with both diffuse gastric cancer and lobular breast cancer |
| 5. Family history of diffuse gastric cancer and lobular breast cancer (one diagnosis before 50 years of age) |
| 6. Detection of signet-ring cells in situ next to diffuse-type gastric cancer |
Gastric cancer predisposition has also been described in the Li–Fraumeni syndrome (due to an underlying p53 mutation), hereditary nonpolyposis colorectal cancer (HNPCC, caused by mutations in DNA mismatch repair genes), Cowden’s syndrome (also known as multiple hamartoma syndrome, caused by PTEN mutation), and Peutz–Jeghers syndrome (hereditary intestinal polyposis, caused by STK11/LKB mutation).
Cancer management
Resectable gastric cancer
The prognosis of patients with gastric cancer remains poor even following radical gastrectomy with a D2 lymph node dissection, with a 5-year survival rate of 31% for stage II and 11% for stage III.19
Surgery
Surgery is critical in the curative treatment of gastric cancer. Endoscopic mucosal resection (EMR) is a minimally invasive procedure offered only to patients with very early gastric cancer (T1a) of intestinal type without ulceration and up to 2 cm in size.20 Annual endoscopic surveillance following treatment is necessary in these patients to detect local recurrence and metachronous gastric cancer.21,22 The incidence of lymph node involvement increases to 20% in tumors involving submucosa (T1b) and these patients require gastrectomy with lymph node dissection.23 The extent of lymph node dissection performed during gastrectomy for gastric cancer has been a matter of controversy. Lymph node dissection is classified according to lymph nodal groups removed during surgery: a D1 dissection involves removal of perigastric lymph nodes directly attached to greater and lesser curvature, D2 involves removal of lymph nodes around the branches of the coeliac axis, and D3 involves removal of additional distant abdominal lymph nodes (at the posterior aspect of the head of the pancreas and at the root of the mesentery).24 The current UICC TNM staging (7th edition) is based upon dissection of 16 or more lymph nodes to assign the pN stage. Two large trials in the West have evaluated the role of extent of surgery by randomizing gastric cancer patients to either a D1 or D2 lymph node dissection. The Medical Research Council (MRC) trial of 400 patients with resectable gastric adenocarcinoma demonstrated equivalent 5-year survival rates in both groups, with increase in postoperative morbidity and mortality reported in the D2 resection arm.19 Pancreatico-splenectomy combined with D2 dissection was demonstrated as an adverse factor affecting survival of patients in the D2 arm in this trial. A more recent Dutch trial of D1 versus D2 dissection demonstrated lower locoregional recurrence and gastric cancer-related death in the patients who underwent a D2 lymphadenectomy with an increase in postoperative mortality and morbidity.25,26 Pancreatico-splenectomy was frequently performed as a part of the D2 lymphadenectomy in this trial and the overall survival (OS) in the D2 group of patients without pancreatectomy or splenectomy has almost doubled (from 5 years in pancreatico-splenectomy D2 group to 9 years in D2 group without organ resection). Further support for the role of radical lymph node resection was demonstrated in a large German gastric cancer study of more than 1600 patients, which reported radical lymph node resection (defined as removal of more than 26 lymph nodes) to be the strongest independent predictor of survival.27 Japanese studies have consistently showed low perioperative mortality with D2 dissection; however, greater morbidity has been reported following more extensive para-aortic nodal dissection without improvement in survival.28
For medically fit patients treated in specialized centers with access to adequate postoperative care, D2 dissection without resection of the pancreas and spleen (unless directly invaded by the tumor) is now the standard in patients with resectable tumors.
Approximately 40% of patients will die as a result of disease recurrence, half of them suffering both local and distant recurrences.25,29 Several approaches incorporating chemotherapy and radiotherapy have been investigated in an attempt to reduce local and distant recurrences and improve the outcome of patients with gastric cancer.
Perioperative chemotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree