Gastric arrhythmias occur in gastroparesis but their significance is debated. An improved understanding is currently emerging, including newly-defined histopathologic abnormalities in gastroparesis. In particular, the observation that interstitial cells of Cajal are depleted and injured provides mechanisms for arrhythmogenesis in gastroparesis. Electrogastrography has been the dominant clinical method of arrhythmia analysis, but is limited by summative nature, low signal quality, and incomplete sensitivity and specificity. Recently, high-resolution (HR; multi-electrode) mapping has emerged, providing superior spatial data on arrhythmic patterns and mechanisms. However, HR mapping is invasive, and low-resolution approaches are being assessed as bridging techniques until endoscopic mapping is achieved.
Key points
- •
The discovery that interstitial cells of Cajal are depleted in gastroparesis provides mechanistic insights into arrhythmogenesis, and reinvigorates clinical interest in gastric electrical testing.
- •
Gastric arrhythmias may play a role in the pathophysiology and symptom generation in gastroparesis, particularly chronic nausea.
- •
Electrogastrography (EGG) has been the dominant clinical method, demonstrating an association between arrhythmias and gastroparesis; however, EGG is summative and has incomplete sensitivity.
- •
The advent of high-resolution (HR; multi-electrode) mapping has been a key advance, and is enabling new insights and classifications.
- •
HR mapping is invasive; low-resolution recordings are being assessed as a bridging method until endoscopic HR recording systems become available for clinical use.
Introduction
Gastroparesis remains a complex clinical and research challenge, with few effective therapies. Among several underlying factors, considerable interest over recent decades has focused on a putative role for gastric electrical arrhythmia. Although the nature and significance of gastric arrhythmia has been a source of considerable controversy, an enhanced understanding is now emerging that could contribute diagnostic and therapeutic value to the problem of gastroparesis.
This review discusses current knowledge of gastric arrhythmia in the context of gastroparesis, and evaluates potential clinical implications in diagnostic testing. Future diagnostic and therapeutic directions for this rapidly evolving field are also considered.
Introduction
Gastroparesis remains a complex clinical and research challenge, with few effective therapies. Among several underlying factors, considerable interest over recent decades has focused on a putative role for gastric electrical arrhythmia. Although the nature and significance of gastric arrhythmia has been a source of considerable controversy, an enhanced understanding is now emerging that could contribute diagnostic and therapeutic value to the problem of gastroparesis.
This review discusses current knowledge of gastric arrhythmia in the context of gastroparesis, and evaluates potential clinical implications in diagnostic testing. Future diagnostic and therapeutic directions for this rapidly evolving field are also considered.
The human “gastric conduction system”
Recent advances in normal human gastric electrophysiology have been significant, such that most textbooks are outdated and a brief overview here is warranted. Although several details await clarification, current progress represents a useful foundation for performing and interpreting tests of gastric electrical function.
Gastric contractions are coordinated by bioelectrical slow waves, which are generated and propagated by networks of interstitial cells of Cajal (ICC). Slow waves are transmitted from ICC to the electrically coupled smooth muscle cells, which generate secondary responses that are integrated with other modulatory factors to effect contractions. In humans, ICC anastomose throughout abundant networks within the gastric myenteric plexus (ICC-MP), as well as running parallel to smooth muscle fibers in the longitudinal and circular muscular layers (intramuscular ICC; ICC-IM). Propagation within these layers is also facilitated by a further “septal” subclass of ICC that encase and connect muscle bundles. Animal studies have shown that a break in ICC continuity occurs at the pylorus, serving as an isolating electrical barrier from the distinct slow wave patterns of the duodenum.
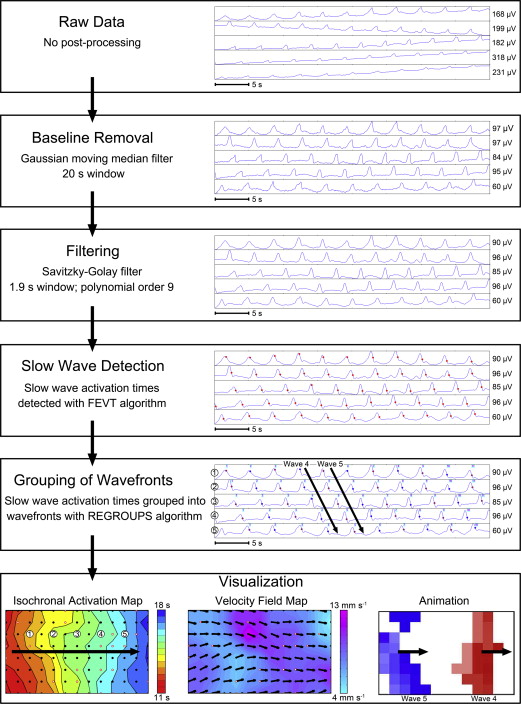
In health, gastric slow waves arise in a defined pacemaker region that is located at the mid to upper corpus of the greater curvature ( Fig. 1 ). Antegrade propagation from this site is facilitated by an underlying frequency gradient within ICC, with cells in the pacemaker region dominating because they have the highest intrinsic frequency. Slow waves propagate toward the pylorus at intervals of around 20 seconds (ie, 3 cycles per minute [cpm]), and at a constant speed of approximately 3 mm/s, such that successive wavefronts become evenly spaced at intervals of around 6 cm. Within the antrum, there is an acceleration in velocity and the wave spacing then becomes greater (see Fig. 1 B). Slow waves do not normally excite the fundus in vivo.
Considerable discussion has focused on what slow wave frequency ranges should be considered normal in humans, with a commonly cited reference range being 2.5 and 3.6 cpm, such that “bradygastric” or “tachygastric” frequencies are defined outside this range. Although these classifications remain useful, particularly for electrogastrography (EGG) interpretation, their importance in humans may have been overstated in the past, because it now seems that much abnormal activity in humans occurs within this normal range.
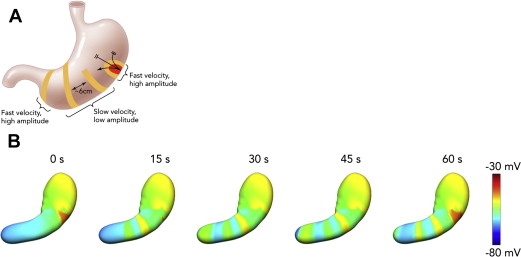
An important, recent finding for arrhythmia analysis has been to clarify the role of velocity anisotropy in normal human gastric conduction. Studies in several species initially documented the presence of rapid conduction in the vicinity of the normal pacemaker, compared with the surrounding corpus (see Fig. 1 ), and it is now known that this occurs because conduction is approximately 2.5 times more rapid in the transverse direction of the human stomach than in the longitudinal (organoaxial) direction. Wavefronts spread out rapidly and circumferentially from the pacemaker; however, by the mid corpus, complete rings of activation have formed such that the rapid circumferential conduction ceases, and the rings thereafter move slowly and longitudinally down the stomach (see Fig. 1 B). Importantly, during gastric arrhythmias, rapid circumferential propagation reemerges to become a major determinant of conduction patterns, as a consequence of either ectopic pacemaking or disruption to the normal ring wavefronts by conduction blocks ( Fig. 2 ).
It is probable that two complimentary gastric conduction pathways are active to explain these anisotropic velocity properties. For example, ICC-MP and ICC-IM might form a bidirectionally coupled network, such that the leading network switches between ICC-MP (dominant during longitudinal conduction) and circular ICC-IM (dominant during circumferential conduction), depending on the presence or absence of complete gastric ring wavefronts. However, this hypothesis awaits experimental validation, and the role of other ICC populations must be clarified.
The pathologic basis for arrhythmias in gastroparesis
Research by the US Gastroparesis Clinical Research Consortium has recently clarified the cellular pathologies underlying idiopathic and diabetic gastroparesis. A reduced density of ICC was found to be the most prominent cellular abnormality in both etiologies, with remaining ICC also showing injury. Other abnormalities were also routinely documented, including a decreased density of nerve fibers, inflammatory infiltrate, and a marked stromal fibrosis. However, the ICC loss may be of particular functional significance to gastroparesis because it has been correlated with delayed gastric emptying in humans, as well as in experimental models.
Given the central role played by ICC in generating and propagating slow waves, these pathologic abnormalities now provide a rational basis for the genesis of arrhythmias in gastroparesis. This concept is also supported by a study showing that the severity of ICC loss correlates with the presence of arrhythmias when assessed by EGG. Although the exact chain of events responsible for these relationships requires further investigation, recent work discussed herein indicates several plausible mechanisms may be contributing.
One helpful classification for understanding gastric arrhythmias, adapted from cardiac rhythm analysis, is to consider them as either “disorders of initiation” or “disorders of conduction” ( Fig. 3 ). Under this schema, it can be surmised that disorders of slow wave initiation result from abnormalities to intrinsic ICC frequencies, whereas disorders of conduction result from disruption to slow wave entrainment through ICC networks.
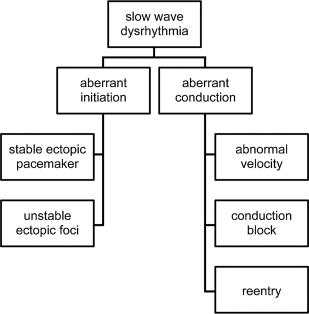
In view of these histopathologic findings recently elucidated in gastroparesis, distinct mechanisms may be proposed to contribute to ICC initiation and conduction abnormalities. In terms of initiation disorders, it is known that nonlethal stressors can promote aberrant frequency responses within ICC, including in humans, adversely affecting intrinsic frequency gradients. Cholinergic regulation is also known to modulate ICC-IM frequencies, potentially inducing arrhythmias, and a role for autonomic neuropathy in aberrant slow wave initiation therefore deserves further investigation. In terms of conduction abnormalities, biophysically based modeling studies have shown that slowed haphazard conduction occurs in states of ICC loss, potentially promoting arrhythmias. In addition, conduction blocks must result when ICC loss falls below a critical threshold. The presence of fibrosis might also be significant; in cardiac electrophysiology, fibrosis is well-known to slow electrical conduction and to play an important role in arrhythmogenesis.
Of clinical interest, another patient group where ICC pathologies may be relevant are those with chronic unexplained nausea and vomiting. It was recently shown that this poorly characterized disease state, in which gastric emptying is normal, shows substantial clinical overlap with gastroparesis, being equivalent in terms of demographics, symptoms, disease duration, health care utilization, and quality of life. Emerging work now further suggests that chronic unexplained nausea and vomiting might even be considered within the same disease category as gastroparesis, because these patients also seem to display reduced ICC and electrical arrhythmias that are similar to those described in gastroparesis. Accumulating evidence points to a threshold of ICC loss at which failure of gastric emptying becomes likely, at around approximately 3 cell bodies per high-powered field within circular muscle, which may be reached in gastroparesis but not in chronic unexplained nausea and vomiting.
These preliminary findings currently await formal publication.
Clinical methods for gastric electrical testing
Electrogastrography
EGG has long been the most widely researched and practiced technique for assessing gastric electrical activity and arrhythmias, involving the placement of cutaneous electrodes over the surface of the epigastrium. A detailed explanation of how to conduct and interpret EGG is available elsewhere.
EGG is clinically attractive owing to its low invasiveness; however, this lack of invasiveness also underlies its several limitations. Because cutaneous signals are recorded at a distance from their gastric sources, they offer only a fundamentally limited and highly summative representation of gastric activation, and it is currently difficult to relate the meaning of these highly integrated signals back to the underlying slow wave events. This problem is further compounded by the fact that 3 wavefronts may be simultaneously active in the human stomach at any 1 time, and possibly more during arrhythmia, which are then summated within a single EGG waveform ( Fig. 4 ).
Despite these considerable limitations, EGG can reliably reflect the underlying dominant slow wave frequency and, for this reason, the primary focus of EGG analyses to date have been in the frequency domain, include analyzing the stability of frequency. Although such data are useful, the recent evidence that human gastric arrhythmias routinely occur at normal frequencies means that frequency-focused approaches may generally underestimate arrhythmia occurrence. Nevertheless, a large number of successful EGG studies have accrued in the literature in recent decades that do consistently demonstrate clear associations between arrhythmic EGG parameters and gastroparesis (eg, Koch and colleagues and Chen and colleagues ).
Despite its diagnostic promise, clinical adoption of EGG has remained weak, with practice limited to a small number of specialist referral and research centers. In addition to the limitations discussed, other factors have contributed to this lack of general clinical interest. The signals are of low amplitude and susceptible to artifacts, such that analyses can be difficult in nonspecialist hands. The test is also perceived as having an incomplete sensitivity and specificity, inconsistent associations with symptoms, and validated protocols defining the role of EGG in clinical algorithms are lacking. The positive predictive value of EGG for normal gastric emptying ranges from 65% to 100% in published studies, versus 50% to 80% for predicting abnormal gastric emptying, although it should be noted that EGG and gastric emptying measure different aspects of motility physiology.
Nevertheless, EGG retains an active and useful role, particularly in research studies, as well as clinically in the screening and diagnosis for motility disorders. The promise and limitations of EGG also continue to motivate the development of improved foundations and clinical methods of conducting the test, for example, by introducing multichannel approaches. However, more accurate and invasive tests of gastric electrical function are much needed to further progress this field.
Extracellular Recordings
Extracellular recordings taken directly from the gastric surface provide the most useful method of analyzing gastric electrical activity in clinical practice. Extracellular recordings may be undertaken at the mucosal or serosal surfaces, classically by using a small number of electrodes placed at sparse intervals or, more recently, by applying dense arrays of electrodes to achieve multi-electrode (high-resolution [HR]) mapping.
The biophysical basis of extracellular recordings was recently addressed, demonstrating robust knowledge of how this technique relates to the underlying electrical activation. Extracellular recordings reflect the localized conduction of slow waves through a defined area of tissue beneath and around the electrode. The potentials approximate the second derivative of the intracellular slow wave time-course, typically adopting a biphasic or triphasic morphology, with the steep negative descent in these signals corresponding with the time of arrival of a wavefront beneath the electrode.
As with EGG, several studies using sparse electrodes have clearly linked arrhythmia with gastroparesis. However, the limitations of such studies must again be appreciated. Single point or linear reconstructions from sparse electrodes give no spatial detail about gastric slow wave patterns, meaning that these analyses have again been largely limited to frequency and rhythm domains, which are not always reliable predictors of arrhythmic onset. Spatial aliasing of sparse data can also lead to misinterpretations.
Mucosal Recordings and “Low-Resolution Mapping”
Clinically relevant extracellular techniques have evolved over the last several decades, particularly approaches to measuring mucosal electrical activity. Different systems to secure mucosal electrodes have been studied, including 1 early system utilizing external rare earth magnets that enabled a comparison of internal (mucosal) and external (cutaneous) recordings simultaneously, providing validity for these approaches.
Some mucosal recording techniques have utilized more than 1 electrode, thus allowing low-resolution reconstructions of propagation to be estimated. More recently, with the advent of temporary gastric electrical stimulation, mucosal recordings, both single point and multiple sites, have been obtained at the time of surgery as part of routine clinical practice. In addition, the same low-resolution approaches can be used serosally, often at the time of gastric stimulator insertion, and with the delivery of energy through proximal to distal electrodes.
High-Resolution Electrical Mapping
In recent years, the limitations with the sparse electrode techniques led Lammers and colleagues to introduce multi-electrode (HR) mapping of slow wave activity. HR mapping involves the placement of dense arrays of many electrodes to track the propagation of slow wave propagation patterns with fine spatiotemporal accuracy. These techniques are well-established in the clinical management of modern cardiac arrhythmias, and are now showing similar potential in the gastroenterology.
Progress in gastrointestinal HR mapping is currently accelerating owing to engineering achievements. These advances include mass producing HR arrays suitable for human applications, and automated methods for analyzing and visualizing the vast volumes of retrieved data. The steps involved in HR mapping analyses are summarized in Fig. 5 .