Fig. 3.1
RoboSurgeon system. Three-dimensional (3D)
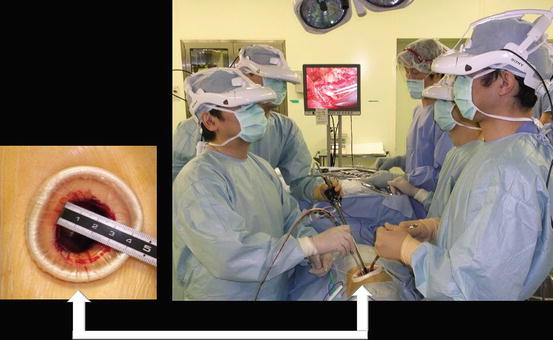
Fig. 3.2
Single port and 3D head-mounted display that all surgeons wear
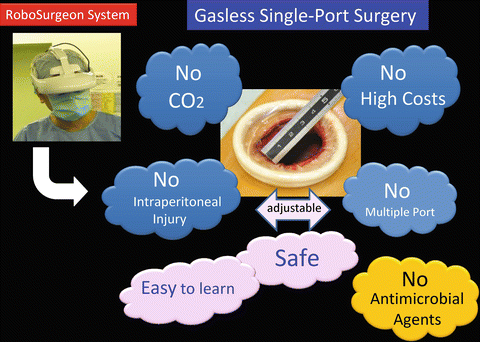
Fig. 3.3
Fundamental concept of gasless single-port RoboSurgeon surgery
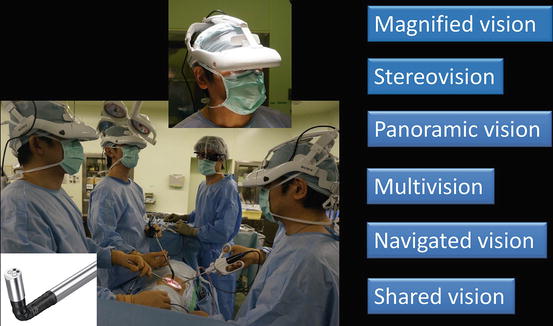
Fig. 3.4
3D-HMD provides six fields of vision in front of the eyes regardless of head position
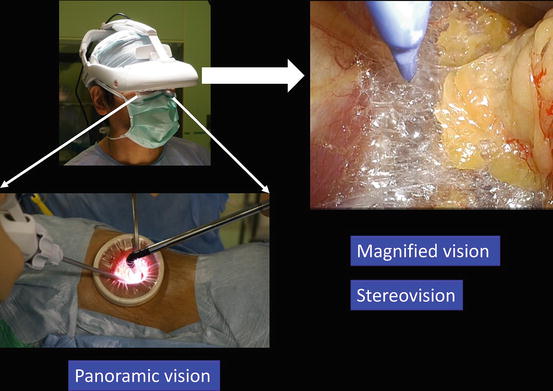
Fig. 3.5
Magnified vision, stereovision, and panoramic vision are obtained. A wide panoramic view can be seen by moving the angle of sight downward
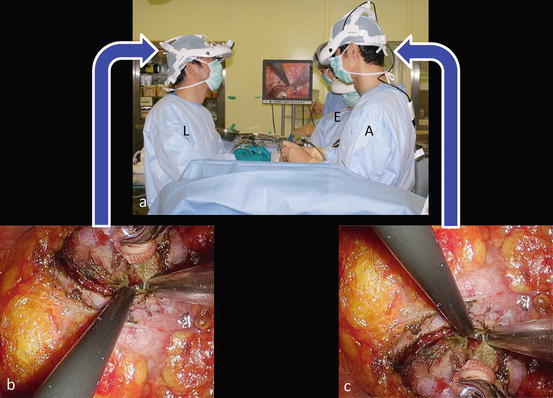
Fig. 3.6
Each surgeon can change the direction of the images on the display according to his or her position
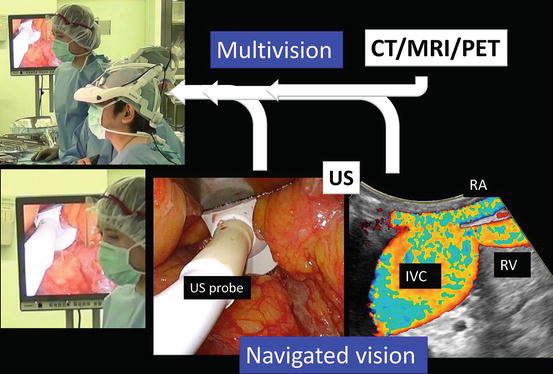
Fig. 3.7
Multivision and navigated vision using ultrasonography via the port. Computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), renal artery (RA), renal vein (RV), ultrasound (US), inferior vena cava (IVC)
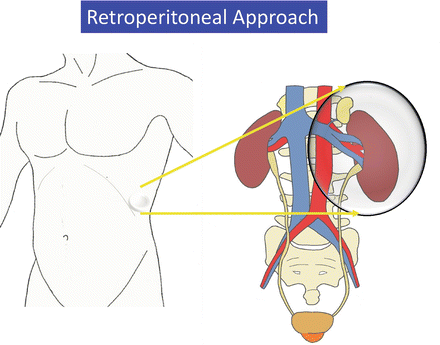
Fig. 3.8
Working space created through a single port
Without the RoboSurgeon system, gasless single-port-like surgery, in which the port size is somewhat larger, can be performed following the procedures presented herein using a system with an ordinary two-dimensional endoscope and display [7, 21]. This surgery may help overcome some of the disadvantages of current laparoscopic and robot-assisted minimally invasive surgeries while retaining their major benefits and adding some unique advantages [22].
3.2 Fundamental Surgical Procedures
The most important steps to accomplish RoboSurgeon clampless partial nephrectomy are (1) mobilization of the kidney so that the tumor can be located just beneath the single port and (2) the mushroom technique, described below, which allows clampless tumor excision and ensures a negative surgical margin (Fig. 3.9). Sufficient mobilization of the kidney allows the tumor to be placed beneath the single port regardless of its location (Fig. 3.10).
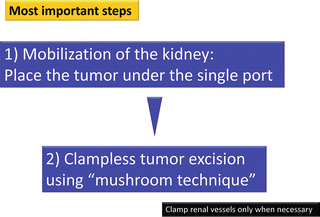
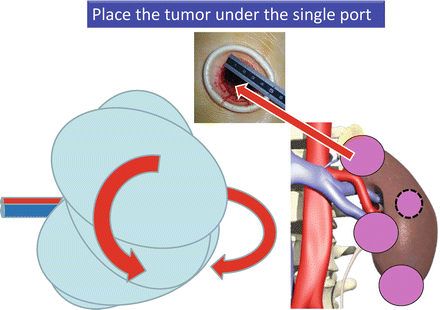

Fig. 3.9
The essential steps for accomplishing RoboSurgeon clampless partial nephrectomy

Fig. 3.10
Mobilization of the kidney. After mobilization of the kidney, almost all partial nephrectomies can be performed just beneath the single port, even in cases of upper pole or medially located tumors
The mushroom technique consists of three steps: (1) coagulating the resection margin and creating a groove around the tumor using an ultrasonic coagulator (Fig. 3.11), (2) tapering the bottom of the groove to create a “mushroom stalk” shape in the tumor base while lifting up the stalk with a thread (Fig. 3.12), and (3) transecting the mushroom stalk within which tumor vessels exist (Fig. 3.13).
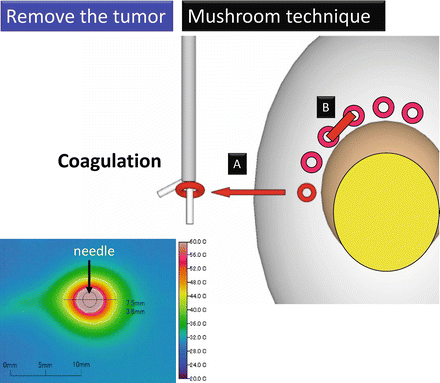
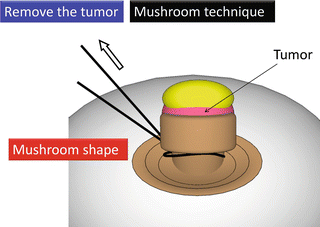
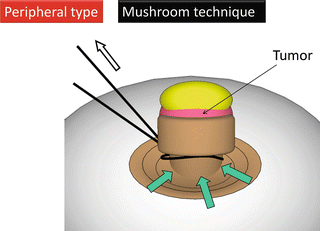

Fig. 3.11
Mushroom technique 1. (a) Coagulate the resection margin. (b) Create a groove around the tumor using ultrasonic coagulating devices

Fig. 3.12
Mushroom technique 2. Create a “mushroom stalk” shape in the tumor base

Fig. 3.13
Mushroom technique 3. Transect the mushroom stalk
Clampless partial nephrectomy does not impose a time limit for tumor resection and renal reconstruction, which facilitates the use of meticulous and precise surgical procedures under the high-quality magnified 3D images of 3D-HMD.
3.3 General Concept
A wide extraperitoneal working space is made along the anatomical plane in the retroperitoneum without gas insufflation and assisted by the RoboSurgeon system that consists of wearable or head movement-controlled devices. For more details, see Chap. 1, Fundamentals of Gasless Single-Port RoboSurgeon Surgery.
3.4 Preoperative Preparation
Patients are provided with sufficient information, including the potential need to extend the port size in the event of difficulties, when obtaining informed consent.
3.5 Instruments
This operation can be performed with reusable and affordable devices. Soft coagulation (VIO300D; ERBE Elektromedizin GmbH) is essentially applied in this operation to stop bleeding via protein degeneration caused by Joule heat. This mechanism enables soft coagulation to stop bleeding securely without vessel wall damage, and it also prevents fistula formation. The endoscope manipulation robot that works with air pressure (Aerovision® [initial version], EMARO® [second version], Riverfield, Inc.) can be replaced by an endoscopist. See Chap. 1 for details.
3.6 Patient Selection
In the initial phase of our experience with the technique, gasless single-port endoscopic partial nephrectomy could be safely performed without vascular clamping and parenchymal sutures in selected cases of well-demarcated, exophytic, and small renal tumors located in the mid-lateral portion or lower pole. According to the progress of the technique described below, various types of renal tumors, approximately 90 % of T1a tumors and some peripheral T1b-T2 tumors, have now been subject to this surgery.
3.7 Surgical Techniques for Peripheral Tumors
3.7.1 Flowchart of the Procedure
A flowchart of the procedure is shown in Fig. 3.14.
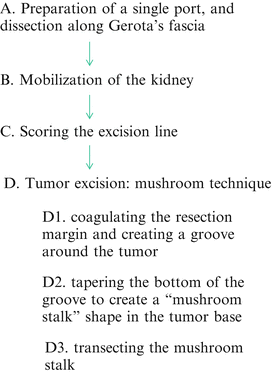

Fig. 3.14
Flowchart of RoboSurgeon gasless single-port partial nephrectomy for peripheral tumors
3.7.2 Patient Positioning
An extraperitoneal flank approach is typically utilized for tumors, irrespective of their location. While under general anesthesia, the patient is placed in the extended flank position (Fig. 3.15). Surgeons put on the 3D-HMD and stand by the table as shown in Fig. 3.16. The lead surgeon operates from the dorsal side of the patient, and the first assistant and the endoscopist (or the endoscope manipulation robot) are located at the abdominal side (Fig. 3.16). The lead surgeon, who is facing the endoscopist, uses reversed images of the endoscope on his or her HMD (Fig. 3.6). Therefore, each surgical team member can operate the endoscopic surgery along his or her axis of vision.
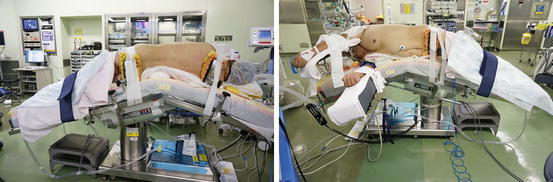
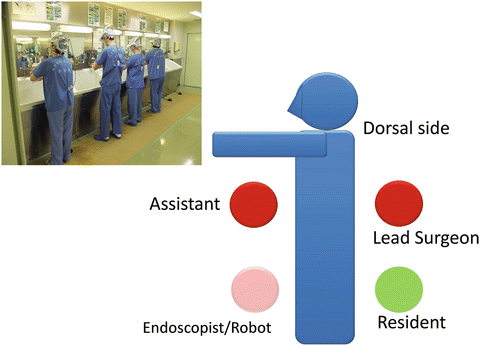

Fig. 3.15
The extended flank position

Fig. 3.16
Surgeon’s position
3.7.3 Preparation of a Single Port, and Dissection Along Gerota’s Fascia
A skin incision of around 4 cm in length is made in the flank, running obliquely near the distal end of the 12th rib (Fig. 3.17a). The size of the single port is adjusted based on the complexity of the tumor and the patient’s body habitus. The tip of the 12th rib is shaved if necessary, and the external and internal oblique muscles are split, while the 12th subcostal neurovascular bundle is spared (Fig. 3.17b). The transverse abdominal aponeurosis is incised (Fig. 3.17c, d).
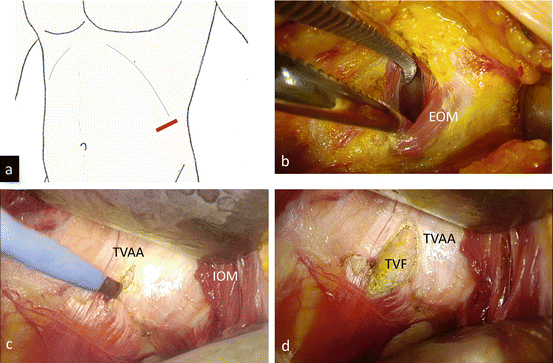

Fig. 3.17
Preparation of a single port. (a) Make a skin incision approximately 4 cm in length in the flank, running obliquely on the distal end of the 12th rib. (b) Shave down the tip of the 12th rib if necessary. Split the external oblique muscle (EOM) and internal oblique muscle (IOM) while the 12th subcostal neurovascular bundle is spared. (c) Incise the transverse abdominal aponeurosis (TVAA). (d) Transversal fascia (TVF) is seen below the TVAA
The retroperitoneal space is opened and perinephric fat is exposed by incising the transversal fascia (Fig. 3.18a), flank pad (Fig. 3.18b), and lateroconal fascia (Fig. 3.18c, d), and this space is dissected sufficiently to create a single port of around 3 cm in diameter using a wound retractor (Alexis wound retractor®, Applied Medical Resources, Corp.) (Fig. 3.19).
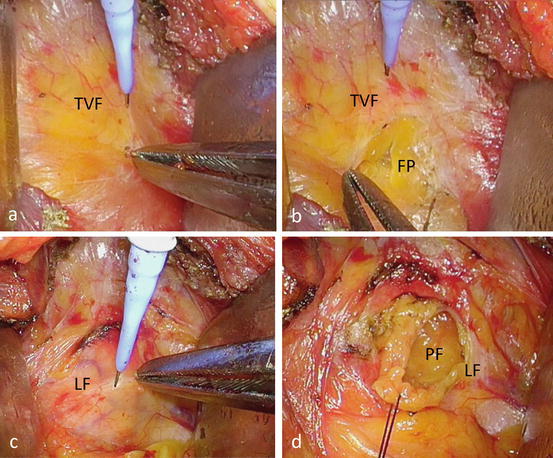
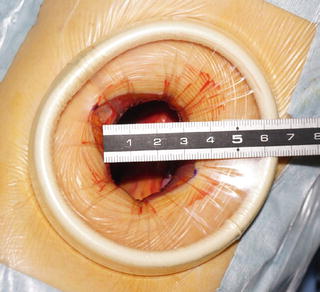

Fig. 3.18
Preparation of a single port (continued). (a–d) Open retroperitoneal space and perinephric fat is exposed by incising transversal fascia (TVF) (a, b), flank pad (FP, b), and lateroconal fascia (LF, c, d)

Fig. 3.19
Preparation of a single port (continued). Dissect RP sufficiently to make a single port of around 3 cm in diameter using the Alexis wound retractor®
3.7.4 Mobilization of the Kidney
The posterior aspect of Gerota’s fascia is dissected from the psoas muscle under 3D magnified vision (Fig. 3.20a). The anterior aspect of Gerota’s fascia is then released from the peritoneum (Fig. 3.20b). The first assistant helps the surgeon create countertraction at the dissection plane (Fig. 3.20c).
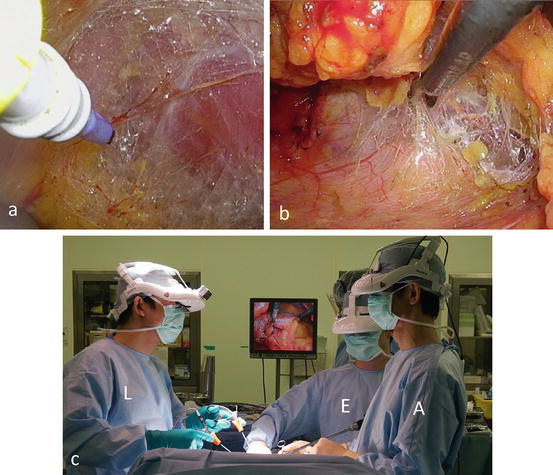

Fig. 3.20
Mobilization of the kidney. (a) Under the endoscopic vision, dissect the posterior aspect of Gerota’s fascia off the psoas muscle. (b) Then release the anterior aspect of Gerota’s fascia from the peritoneum. (c) The first assistant (A) helps the surgeon to create countertraction at the dissection plane. Lead surgeon (L), first assistant (A), endoscopist (E)
Gerota’s fascia is incised, and the kidney is mobilized in the perinephric fat (Fig. 3.21), which allows for partial nephrectomy just beneath the single port, even in cases of upper pole or medially located tumors (Fig. 3.10).
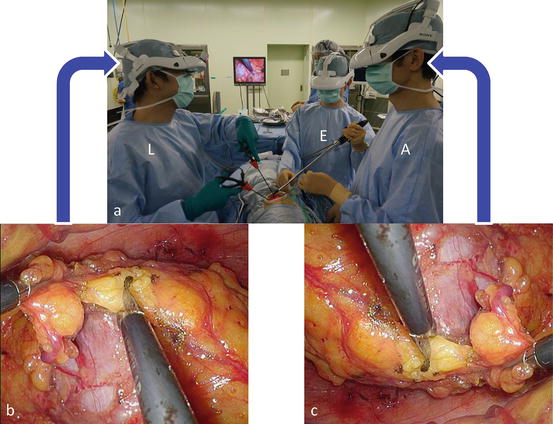

Fig. 3.21
Mobilization of the kidney (continued). (a) Incise Gerota’s fascia and mobilize the kidney in the perinephric fat. The first assistant (A) helps the surgeon to create countertraction at the dissection plane. (b) The lead surgeon (L) uses reversed images of the endoscope on his or her head-mounted display. (c) The first assistant (A) and the endoscopist (E) use the original image. Each surgical team member can operate the endoscopic procedure along his or her axis of vision
3.7.5 Scoring the Excision Line Under the Guidance of Ultrasonography
Intraoperative ultrasonography is performed to define the extent and depth of the tumor and to score the proposed excision line with a 5-mm margin (Fig. 3.22a). The resection margin around the tumor is scored on the renal capsule with electrocautery (Fig. 3.22b, c). Color Doppler ultrasonography is used to identify intraparenchymal tumor vessels, which are finally transected (Fig. 3.22a).
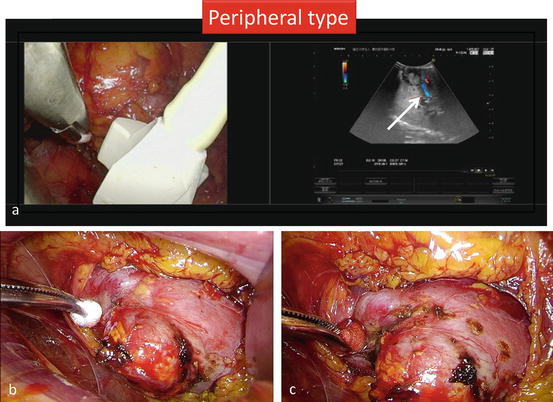

Fig. 3.22
Scoring the excision line under the guidance of ultrasonography. (a) Perform intraoperative ultrasonography to define the extent and depth of the tumor and to score the proposed excision line with a 5-mm margin. Color Doppler ultrasonography is used to identify intraparenchymal tumor vessels, which are finally transected. Using a two-split screen, each surgeon continuously and simultaneously views the endoscopic and ultrasound images. (b, c) Score the resection margin around the tumor on the renal capsule with electrocautery
3.7.6 Coagulating the Resection Margin and Creating a Groove Around the Tumor
An active blade of an ultrasonic coagulator (Harmonic®, Ethicon) is inserted into the normal parenchyma along the proposed resection margin at intervals of approximately 7 mm (Figs. 3.11 and 3.23). Ultrasonic coagulation for 40–50 s degenerates the renal parenchyma within a diameter of approximately 7.5 mm (Figs. 3.11 and 3.23c).
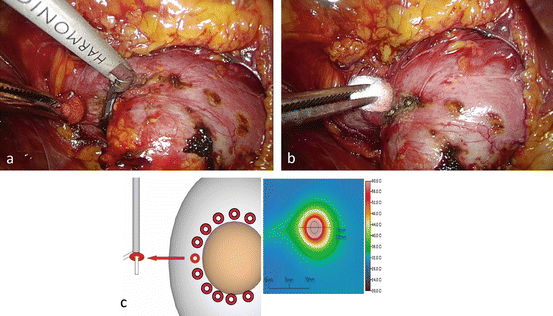

Fig. 3.23
Coagulating the resection margin and creating a groove around the tumor using ultrasonic coagulating devices. (a, b) Insert an active blade of an ultrasonic coagulating device into the normal parenchyma along the proposed resection margin at intervals of 7 mm. (c) Ultrasonic coagulation for 40 s degenerates the renal parenchyma within a diameter of 7.5 mm
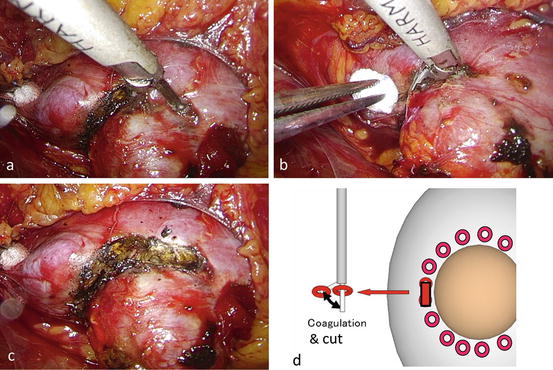
The renal parenchyma between each coagulated site is then cut with the ultrasonic coagulator to create a groove around the tumor (Fig. 3.24).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








