Fig. 1.1
Fundamental concept of gasless single-port RoboSurgeon surgery
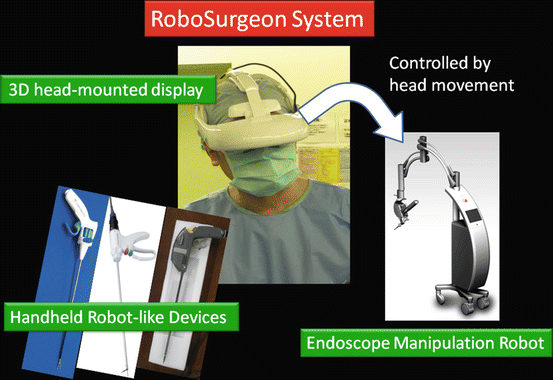
Fig. 1.2
RoboSurgeon system. Three dimensional (3D)
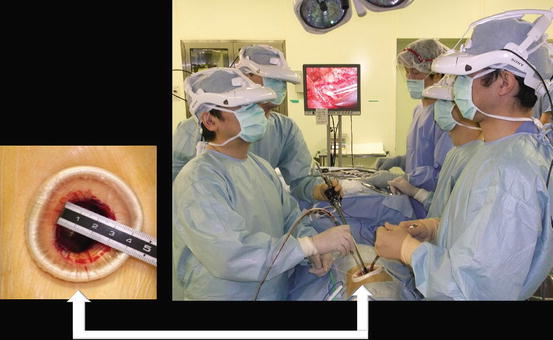
Fig. 1.3
Single port and 3D head-mounted display that all surgeons wear
This surgery may overcome some of the disadvantages of existing laparoscopic and robotic minimally invasive surgeries, while retaining their major benefits and possibly adding some unique advantages.
1.2 General Concept
This surgery is carried out in the wide retroperitoneal working space made along the anatomical plane without gas insufflation, through a roughly coin-sized single port, assisted by a system (RoboSurgeon) that involves the robotization of the surgeon (Figs. 1.1, 1.2, 1.3, and 1.4).
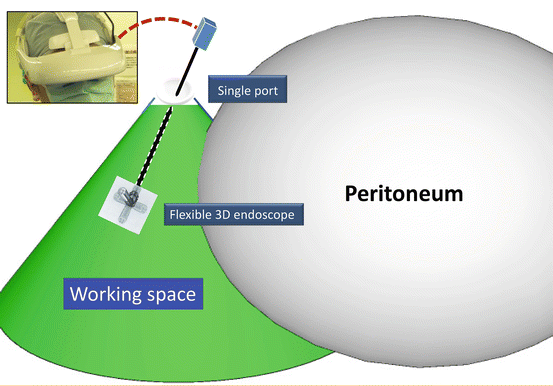

Fig. 1.4
Wide retroperitoneal working space
Performing this surgery achieves four key “nos”: no high costs, no CO2, no multiple ports, and no intraperitoneal injury (Fig. 1.5).
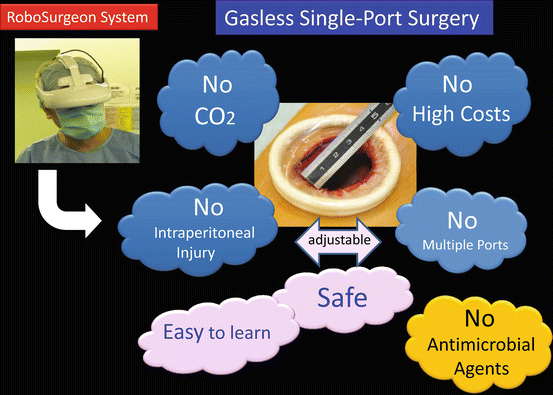

Fig. 1.5
Key features of gasless single-port surgery
High costs are avoidable because most of the devices are reusable, and highly expensive instruments are not necessary. The cost of surgery is nearly the same as that of open surgery, and hospitalization expenses are nearly the same as those associated with laparoscopic or robot-assisted surgery.
Adverse effects due to CO2 insufflation are prevented (Table 1.1), and careful care required by the anesthesiologist during operation is minimized. For patients with underlying low cardiopulmonary or renal function, no CO2 insufflation may be preferable. The CO2-free nature of the surgery may create an eco-friendly association from the standpoint of global CO2 elevation.
Table 1.1
Adverse effects due to CO2 insufflation
Hypercapnia |
Hypertension/hypotension |
Decline of lung compliance |
Reduction of urine volume |
Gas embolism |
Subcutaneous emphysema |
Shoulder pain |
One lung intubation |
Adverse effects of the transperitoneal approach (e.g., postoperative intestinal obstruction) are avoided, and patients with extensive prior abdominal surgery can undergo this procedure.
No prophylactic antimicrobial agents are needed in most of the operations due to the minimal incision, effective washing of the operative field, effective protection of the edge of the incision, and no insertion of fingers, which may contribute to reduction of drug-resistant bacteria [15–18].
Safety is also guaranteed by easy, instant, and precise inch-by-inch extension of the port size tailored to the patient’s situation, including, but not limited to, obesity, performance status, comorbidity, or the operator’s experience. Conversion to open surgery, which almost never occurs, can also be accomplished swiftly. Based on these safety features, learning this operation is rather easy, as described below.
1.3 Gasless Single-Port Surgery
1.3.1 Approach and Positioning of the Patient
The retroperitoneal approach through a flank port in the flank position is the standard in surgeries for the adrenal gland, kidney, and upper urinary tract (Fig. 1.6). In cases with low cardiopulmonary function, severe osteoporosis, and horseshoe kidney and for cosmetic purposes, the retroperitoneal approach through a pararectal port (Chap. 2) or an umbilical port (Chaps. 3 and 4) in a supine position is chosen (Figs. 1.7 and 1.8). For a large pheochromocytoma, the transperitoneal approach, which usually spares muscles, is frequently selected (Fig. 1.7 and Chap. 5).
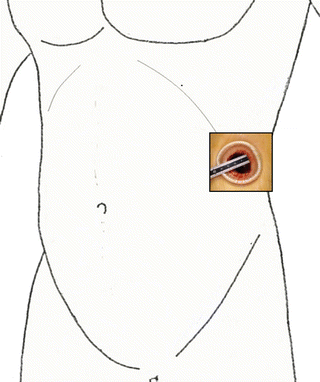
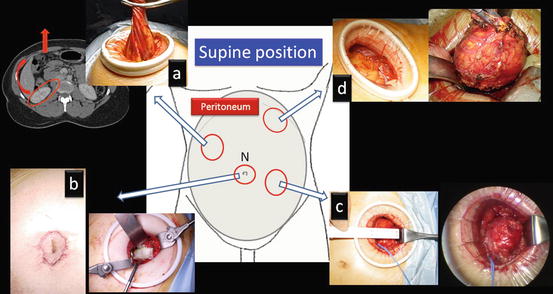
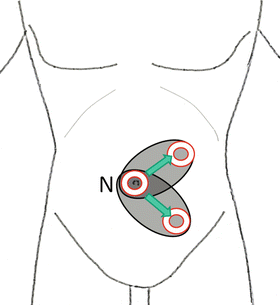

Fig. 1.6
Standard port site for the adrenal gland, kidney, and upper urinary tract

Fig. 1.7
Various retroperitoneal approaches through a single-port in the supine position. (a) Pararectal retroperitoneal radical nephrectomy; (b) transnavel retroperitoneal partial nephrectomy; (c) pararectal retroperitoneal partial nephrectomy; and (d) transperitoneal adrenalectomy for large pheochromocytoma

Fig. 1.8
Transnavel retroperitoneal approach. The skin hole at the navel site is shifted to the pararectal portion where a single-port is made. Navel (N)
As for intrapelvic organs, the retroperitoneal approach through a lower abdominal port in a supine position is used (Fig. 1.9).
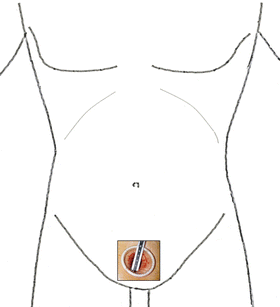

Fig. 1.9
Standard port site for intrapelvic organs
1.3.2 Working Space
A wide working space is created extraperitoneally through a single port as an initial step by creating separation along the anatomical plane. For the adrenal gland, kidney, and upper urinary tract, the space is made by dissecting along Gerota’s fascia, usually through a port around the tip of the 12th rib (Figs. 1.10 and 1.11).
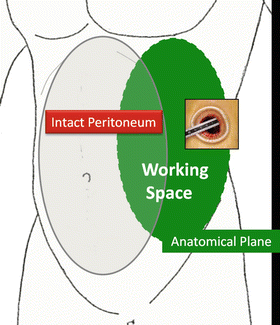
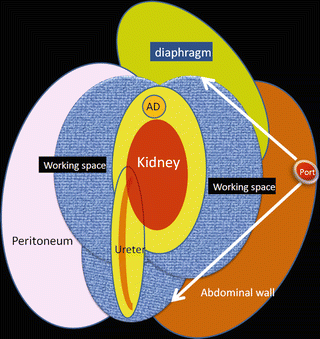

Fig. 1.10
Working space in the adrenal gland, kidney, and upper urinary tract surgeries

Fig. 1.11
Working space (shown in blue) made through a flank port along Gerota’s fascia. Adrenal gland (AD)
For the intrapelvic organs (i.e., prostate, bladder, and pelvic lymph nodes), the working space is created by dissecting along the fascia covering the perivesical fat through a port in the lower abdomen (Figs. 1.12 and 1.13).
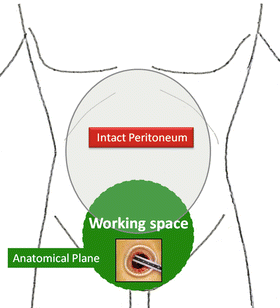
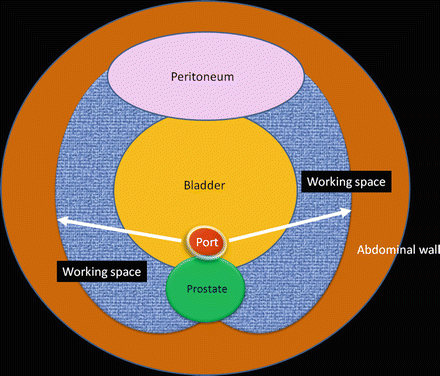

Fig. 1.12
Working space in the intrapelvic organ surgeries

Fig. 1.13
Working space (shown in blue) made through a single port in the lower abdomen along the perivesical fatty tissue
When both working spaces are required, such as in nephroureterectomy, two single-port surgeries are combined (Fig. 1.14).
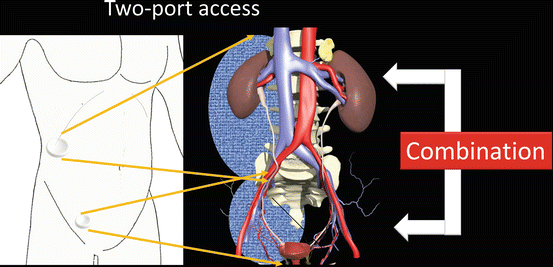

Fig. 1.14
Combination of the two single-port surgeries
1.3.3 Single Port
The standard size of the single port in the flank or the lower abdomen is usually around 2–4 cm, approximately the size of a coin. The sizes for major urologic surgeries are presented in Fig. 1.15. The port size is adjusted based on the patient’s situation and occasionally on the surgeon’s experience. Precise inch-by-inch adjustments are possible before or during the operation. Through the port, which can be extended to facilitate extraction, the targeted specimen is extracted as shown in Fig. 1.16.

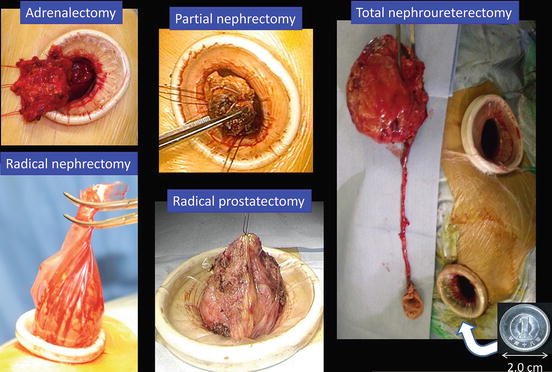

Fig. 1.15
Standard sizes of the single port for major urological surgeries

Fig. 1.16
Typical images of extraction of the specimen through a port
1.4 RoboSurgeon System
The RoboSurgeon system comprises three components: a 3D-HMD, handheld robot-like devices, and an endoscope manipulation robot that works with air pressure (Aerovision® [initial version], EMARO® [second version], Riverfield, Inc.).
1.4.1 Three-Dimensional Head-Mounted Display
The 3D-HMD for medical use (SONY Corp.) has 1,280 × 720 resolution from the two 0.7-in. organic light emitting diode (OLED) panels that process 256 levels colors each for red, green, and blue (Fig. 1.17). Separate OLED display panels for left and right eyes offer a smooth, natural 3D view. The 3D-HMD is connected to an image processor to provide clear, high-quality 3D images in front of the eyes regardless of head position (Figs. 1.17 and 1.18). Via the processor, the 3D-HMD is connected to a flexible (Olympus) or a thin (Shinko Optical) 3D endoscope (Fig. 1.18). The digital zoom function works by pressing a button on the 3D flexible endoscope (Fig. 1.19).
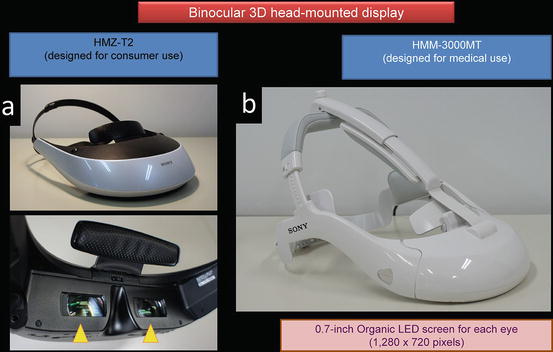
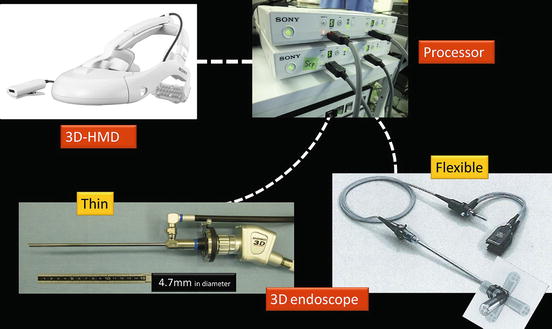
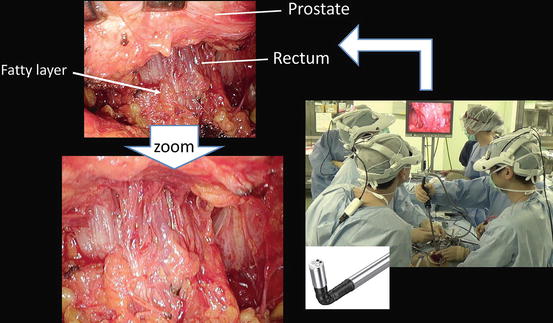

Fig. 1.17
3D-HMD for (a) consumer use and for (b) medical use modified from (a)

Fig. 1.18
3D-HMD is connected to a flexible (Olympus) or a thin 3D-endoscope (Shinko Optical)

Fig. 1.19
Digital zoom function works by pressing a button on the flexible 3D endoscope
The 3D-HMD provides six fields of vision (magnified vision, stereovision, panoramic vision, multivision, navigated vision, and shared vision) (Fig. 1.20). Clear magnified stereovision enables the surgeon to perform complex maneuvers, especially in deep sites (Fig. 1.21). Panoramic vision (direct vision), which is obtained by moving the angle of sight downward, is helpful in maintaining safety throughout the operation. Each surgeon can change the direction of the images on the display according to his or her position and share a common direction with other surgeons (Figs. 1.22 and 1.23).
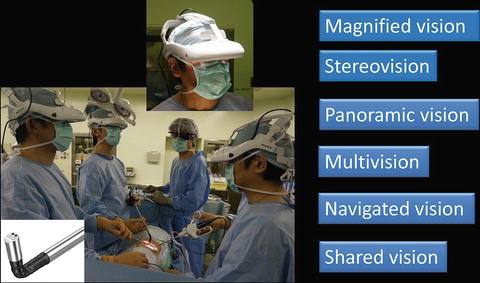
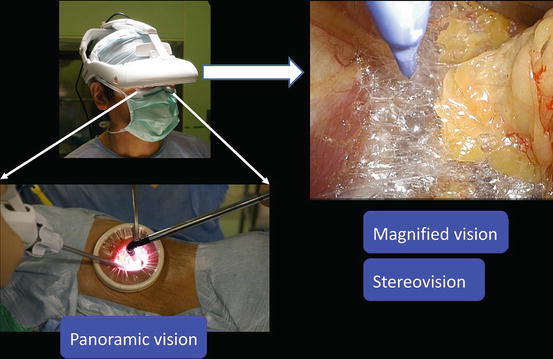
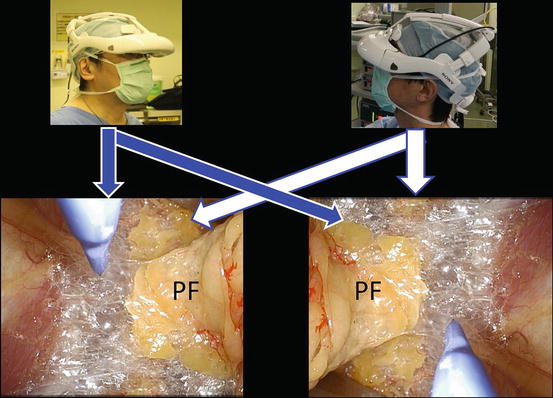
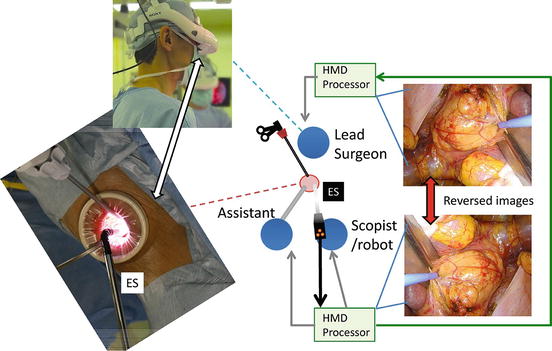

Fig. 1.20
3D-HMD provides six fields of visions in front of the eyes, regardless of head position

Fig. 1.21
Magnified stereovision and panoramic vision obtained by moving the angle of sight downwards

Fig. 1.22
Each surgeon can change the direction of the images on the display according to his or her position. Perinephric fat (PF)

Fig. 1.23
The endoscopist is positioned opposite the lead surgeon. The images on the lead surgeon’s display are reversed from those on the endoscopist’s display
Various images of X-ray, ultrasound, computed tomography, magnetic resonance imaging, positron emission tomography, etc. are seen on the 3D-HMD (Fig. 1.24). Navigation using ultrasonography via the port or the rectum aids the process (Figs. 1.24 and 1.25). Two images in the original set, and four images when a multiplexer is applied, are seen simultaneously on the 3D-HMD (Fig. 1.26). Images are shared among all participating surgeons. The images can be controlled by a foot switch or via finger movement when a camera is set on the HMD (Figs. 1.27 and 1.28).
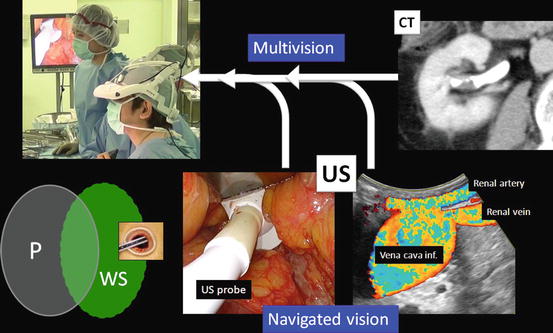
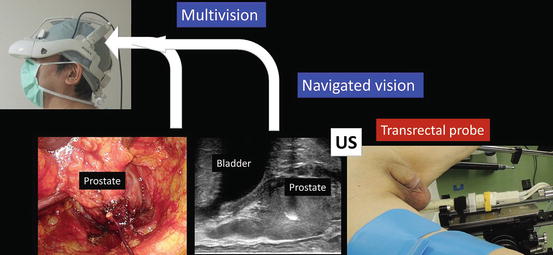
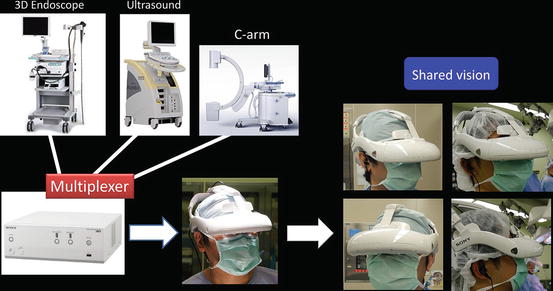
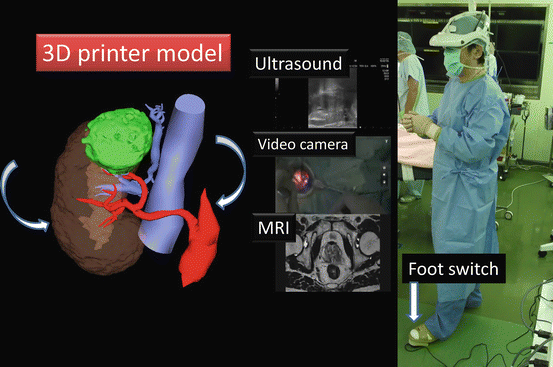
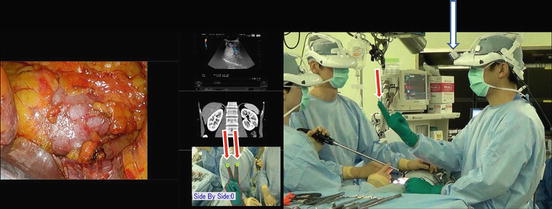

Fig. 1.24
Multivision and navigated vision using ultrasonography via the port. Peritoneum (P); working space (WS)

Fig. 1.25
Navigated vision using transrectal ultrasonography (US) in intrapelvic organ surgeries

Fig. 1.26
Various images displayed on the 3D-HMD using the multiplexer are shared among all participants

Fig. 1.27
Image control with a foot pedal. Magnetic resonance image (MRI)

Fig. 1.28
Image control by finger movement (red arrow). Camera on the display (white arrow)
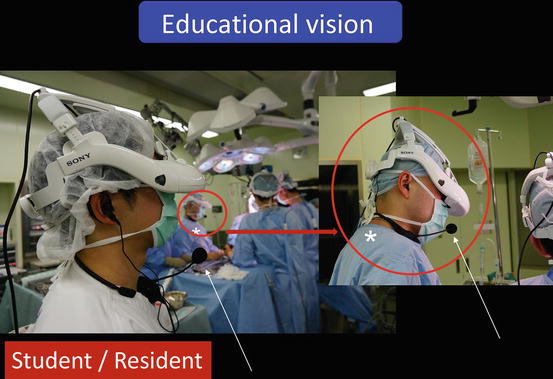
Instructors, students, nurses, or others who instruct or learn from the operation can see the same images as the surgeon and communicate with him or her directly (Fig. 1.29). They can also view these images and communicate with the surgeon from another room (Fig. 1.30).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








