Frequently Asked Questions
When is MRI preferable to CT for imaging the liver, kidneys, or pancreas?
Many patients in whom iodinated contrast agents are contraindicated are referred for magnetic resonance imaging (MRI) instead of computed tomography (CT) for cross-sectional imaging. The usual contraindications to iodinated contrast agents include renal insufficiency or a history of a prior moderate-to-severe allergic reaction to these agents. Patients may also be diverted from CT to MRI when residual barium from a prior gastrointestinal study remains in the bowel. Barium can cause severe artifacts on a CT scan but produces little or no distortion on abdominal MR images. MRI is also preferable to CT for assessing iron-deposition disorders and fatty infiltration or sparing of the liver or pancreas. With the widespread availability of high-quality magnetic resonance cholangiopancreatography (MRCP) sequences, MRI offers superior evaluation of biliary and pancreatic duct disease.
The currently available gadolinium chelates have biodistribution properties that are almost identical to iodinated contrast agents. Like iodinated agents, they are distributed in the extracellular fluid space and are eliminated primarily via glomerular filtration. For this reason, a dynamic multiphase MRI examination is unlikely to yield more information than a dynamic multiphase CT scan in terms of lesion enhancement characteristics. The advantage of MRI over CT is the ability to further characterize tissue with chemical shift imaging or T1 and T2 relaxation times.
MRI requires a greater degree of patient cooperation than CT, and severely ill patients may not tolerate the longer imaging times of MRI. Local expertise is another factor in determining whether a patient should be referred for CT or MRI. In most cases, a well-performed and expertly interpreted CT scan is preferable to a poorly performed and interpreted MR scan.
Which is better, CTA or MRA?
Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) are equally efficacious in assessing many vascular abnormalities of the abdomen and pelvis. When choosing between these techniques, one should consider several factors. These include the availability of equipment and expertise, history of contrast allergy, renal insufficiency, or claustrophobia, the size of the patient, and the presence of implanted devices, which might cause the patient harm or result in unacceptable image artifact.
MRA has the advantage of potentially providing quantitative flow data, although this is not widely used. If performed at a standard clinical dose (0.1 mmol/kg), contrast-enhanced MRA may be repeated, if necessary, without fear of nephrotoxicity. Noncontrast-enhanced MRA techniques can be repeated as often as necessary to obtain an efficacious result.
When should patients be kept NPO before performing abdominal MRI?
It is not necessary to keep patients NPO (given nothing by mouth) for most MRI examinations. Nausea and vomiting are rarely encountered with gadolinium administration, and intestinal contents rarely interfere with diagnosis. Limiting oral intake before MRCP may be helpful to reduce gastric and small bowel signal, which may interfere with thick-slab images of the biliary and pancreatic ducts. However, creative scan plane acquisition can also be used to overcome this problem. Intestinal contents are frequently bright on three-dimensional (3D) gradient echo sequences used for MRA and may degrade the quality of 3D reconstructions. Although dietary restrictions may reduce this signal, much of this signal can also be eliminated with subtraction techniques. A few MRI techniques, such as MR colonography, do require dietary restrictions, but they are not widely performed.
Is it useful to administer glucagon for abdominal MRI?
Some experts recommend administration of glucagon for imaging the bowel and peritoneum. As MR sequences become faster, the need for glucagon even in these settings will likely diminish. The only situation in which we routinely administer glucagon is when imaging with an endorectal coil. In this case, 1 mg of glucagon injected intravenously just before imaging reduces rectal contractions that can seriously degrade image quality.
Which sequence is most sensitive for lesion detection in the liver?
No single sequence is 100% sensitive for the detection of liver lesions. In addition, the sensitivity of some sequences may be enhanced with the use of intravenous contrast agents. In the case of routine imaging, dynamic gadolinium-enhanced fat-suppressed T1-weighted images are currently the most sensitive. However, other sequences may occasionally demonstrate lesions not seen on the dynamic series. The sensitivity of T2-weighted sequences varies greatly. A respiratory-triggered fat-suppressed T2-weighted fast spin echo sequence in a cooperative patient detects more lesions than a breath-hold fast spin echo sequence in an uncooperative patient. In addition, sensitivity depends in part on the type of lesion imaged. For example, HASTE sequences have very high sensitivity for detecting benign lesions such as cysts and hemangiomas. However, the sensitivity for detecting malignant tumors with these techniques is considerably less. Finally, the sensitivity of T1-weighted and T2-weighted imaging can be enhanced for some lesions with the addition of mangafodipir and ferumoxides respectively, although these agents are only used selectively at most centers.
What is the best T2-weighted sequence for abdominal and pelvic MRI?
A variety of T2-weighted sequences can be used to evaluate the abdomen and pelvis. Most centers use a T2-weighted fast spin echo sequence for routine imaging. However, these sequences typically have scan times of several minutes. As a result, they cannot be performed during suspended respiration without significant modification, and image quality may be poor in uncooperative patients. HASTE sequences perform well when motion is a problem, in the presence of ascites, and when imaging fluid-containing structures (e.g., for MRCP and MR urography). In addition, these sequences are relatively resistant to susceptibility artifact. However, image blurring and a relatively low signal-to-noise ratio limit these sequences. In addition, many MR practitioners have noted subjectively decreased solid tumor conspicuity with these sequences.
Preliminary evidence suggests GRASE imaging performs similarly to fast spin echo imaging in the abdomen, although clinical experience with this sequence is limited. Scan times tend to be shorter with GRASE. This sequence has the additional advantage of reducing the specific absorption rate compared with fast spin echo, an important benefit when imaging at field strengths greater than 1.5 T. Steady-state gradient echo sequences, such as trueFISP or balanced FFE, allow for rapid production of images that appear T2-weighted, although the actual image contrast is T2/T1-weighted.
Can T2-weighted imaging be performed after gadolinium administration?
In most instances, the answer is yes. Structures that concentrate gadolinium, such as the renal collecting systems, demonstrate signal loss resulting from the T2 shortening effects of gadolinium. However, most structures and organs do not concentrate gadolinium chelate sufficiently for this effect to be of significant concern. There is some evidence that the conspicuity of solid liver lesions improves on T2-weighted images performed after the administration of intravenous gadolinium (1).
Should I be using 2D or 3D dynamic contrast-enhanced imaging of the abdomen?
In general, stick with what works best on a particular scanner. A well-executed dynamic two-dimensional (2D) scan is clearly preferable to a poor quality 3D scan. In general, though, there is a steady trend toward 3D dynamic imaging for most abdominal and pelvic applications. There are three main advantages of 3D acquisitions: (1) the thin effective slice thickness (2 to 3 mm) improves through-plane resolution; (2) isotropic or near-isotropic voxels facilitate multiplanar reconstruction in any plane; and (3) arterial and venous phase images provide an excellent depiction of the abdominal and pelvic vasculature.
Should I use a gradient echo or spin echo technique for T1-weighted imaging of the abdomen and pelvis?
Gradient echo imaging is preferable any time breath-hold imaging is advantageous. As a result, gradient echo imaging has largely replaced spin echo imaging for most abdominal applications. Spin echo and fast spin echo imaging are used more commonly in the pelvis where respiratory motion is not as problematic. In addition, these latter sequences provide for higher resolution imaging with improved signal-to-noise ratio.
If an adrenal mass is indeterminate on noncontrast CT, what is the likelihood that MRI will be diagnostic for adenoma?
In-phase and opposed-phase MRI and noncontrast CT rely on the same histologic feature to characterize adrenal masses (2). With both techniques, detection of a significant concentration of intracellular lipid leads to a diagnosis of adenoma. Adrenal masses with insignificant intracellular lipid are considered to represent other types of adrenal masses or lipid-poor adenomas. Therefore, it is unlikely that an MRI study will be helpful if the noncontrast CT scan is indeterminate. However, the answer depends partly on the Hounsfield threshold used to characterize the adrenal mass with CT. If one sets the CT threshold low (e.g., less than 10 HU), some adrenal masses considered indeterminate by CT may demonstrate signal loss on opposed-phase images.
Does an entire adrenal mass need to lose signal on opposed-phase imaging to call it an adenoma?
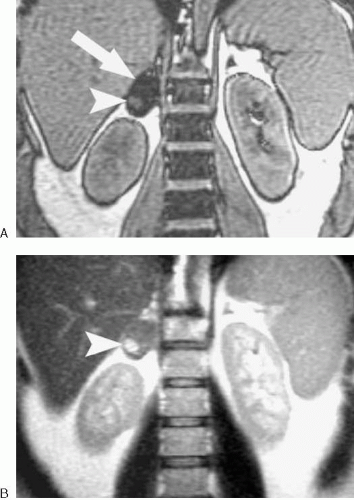
Although most adrenal adenomas demonstrate homogeneous signal loss on opposed-phase images, some do not. Heterogeneous signal loss can occur when some portions of an adenoma contain more lipid than others. In addition, adenomas complicated by hemorrhage can contain areas that do not “drop out” on opposed-phase images (Fig. 4.15).
There is an entity, referred to as a collision tumor, in which a metastatic focus exists in or is contiguous with an adrenal
adenoma (Fig. 4.16) (3). This is a very unusual finding and is considerably less common than an adenoma with hemorrhage or variable lipid content. However, any area of heterogeneity that appears masslike should be viewed with suspicion, particularly if the patient has a known malignant tumor. Sometimes comparison with prior examinations reveals that the mass has either grown over time or remained stable for several years, improving diagnostic confidence.
adenoma (Fig. 4.16) (3). This is a very unusual finding and is considerably less common than an adenoma with hemorrhage or variable lipid content. However, any area of heterogeneity that appears masslike should be viewed with suspicion, particularly if the patient has a known malignant tumor. Sometimes comparison with prior examinations reveals that the mass has either grown over time or remained stable for several years, improving diagnostic confidence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree