Never
Rarely
Sometimes
Weekly
Daily
Incontinence for solid stool
0
1
2
3
4
Incontinence for liquid stool
0
1
2
3
4
Incontinence for gas
0
1
2
3
4
Alteration in lifestyle
0
1
2
3
4
No
Yes
Need to wear a pad or plug
0
2
Taking constipating medicines
0
2
Lack of ability to defer defecation for 15 min
0
4
The Anatomy of Incontinence
Historically, Milligan and Morgan suggested the anorectal ring was key to continence following fistulotomy, indicating as long as a complete ring of muscle is left intact ‘all the anal sphincter muscles below this ring may be divided […] without harmful loss of control’ [5]; but social niceties were likely different in those days. Impairment of continence following fistulotomy is well recognised and factors affecting this impairment are increasingly understood.
Whereas the external sphincter was once considered the more important in maintaining continence, we have argued recently that internal sphincter division, seen when even low fistulas are laid open, determines most functional disturbance after either high or low fistulotomy—except where Milligan and Morgan’s anorectal ring is cut, when frank and devastating incontinence will result. Others dispute this [6, 7].
Our two studies examined incontinence following fistulotomy in patients who underwent either internal sphincterotomy for intersphincteric tracts or internal and external sphincterotomy for transsphincteric tracts and demonstrated a similar level of minor continence disturbance [8, 9]. In addition, anal manometry following sphincterotomy was performed in the Lunniss et al. study which found that while all patients undergoing fistulotomy had a reduced maximum resting anal tone, there was no difference in this reduction after division of both sphincters when compared with IAS division alone [9]. Division of the EAS did lower squeeze pressures in the lower canal whereas IAS division alone did not, but without functional consequence. Combining the manometric and clinical data suggests that IAS division, which reduced maximum resting anal tone, was associated with a minor impairment of continence, whereas additional division of the EAS reduced the voluntary squeeze pressure but did not influence functional outcome. Complete division of the EAS was not performed in any patients. Higher thresholds of anal electrosensitivity in the area of the divided anoderm were also noted.
Similar findings in 148 patients who underwent fistulotomy for intersphincteric fistulas (and therefore IAS division alone) were published by Toyanaga et al. in 2007. They found that resting tone and length of the high pressure zone were reduced following fistulotomy but voluntary contraction was not affected and of the 30 patients (21 %) who suffered impairment of continence, only four suffered a higher degree than flatus incontinence [10]. Likewise Chang et al. found similar manometric results in a study of 45 patients who underwent fistulotomy for intersphincteric tracts in whom resting pressures were reduced but voluntary squeeze was unaffected [11].
Further support for the idea that IAS division is the main factor leading to minor disturbance in bowel control after fistulotomy comes from Kennedy et al. who described a technique to preserve the EAS by encircling it with a seton after laying open the fistula through the IAS [12]. Despite a completely preserved EAS, at least a third of patients developed minor incontinence.
Internal sphincterotomy for other conditions, such as fissure, also disturbs continence. Bennett and Goligher in 1962 found that 34 % of 127 patients undergoing internal sphincterotomy for fissure suffered flatus incontinence, although this rate diminished with longer follow-up [13]. In another study published in 1989, Khubchandani et al. found impairment of continence in as many as 35 % of patients who had undergone sphincterotomy for fissure [14]. Refinements to the technique, including the position and length of the sphincterotomy as well as more careful selection (given effective medical alternatives), have seen incontinence rates falling in recent reports, but the association between IAS injury and minor incontinence remains clear.
In a study of patterns of incontinence after anal surgery (including sphincterotomy, fistulotomy and others), Lindsey et al. examined 93 patients with incontinence and considered the nature of their sphincteric injuries [15]. Ninety-eight percent of the patients had an IAS injury on endoanal ultrasound, whereas only a third had an EAS injury. Most patients had defects in the high pressure zone of the anal canal which led to a reverse of the normal resting pressure gradient.
In addition to the quantity of muscle divided, Zbar has argued that specific factors, such as the rectoanal inhibitory reflex or a recognisable distal sphincter deficiency noted in some patients before surgery, as well as other measurable parameters from anal manometry or MR imaging, may all contribute to incontinence after surgery and that assessment of these factors may reduce predictable post-operative functional impairment summarised in [16], adding that preservation of the IAS is vital for pristine continence. However, without an evidence-based systematic approach to preoperative anal sphincter assessment, perhaps using manometry and imaging (but see below), and without an understanding of the influence of such an approach on the outcomes of surgery, the place of these techniques in preoperative decision making is unclear. What is more, for all its apparent objectivity anorectal physiology testing is not that objective: the same test repeated by the same operator on different occasions gives different results; the same test by different operators on the same occasion gives different results.
Assessment Before Fistulotomy
In the office/outpatient setting assessment of pre-existing bowel function and continence is important, along with a history of previous sphincter surgery or potential injury (e.g. complicated vaginal delivery). IBS is a relative contraindication to lay open.
The key is the ability to feel the internal opening in a conscious patient and to estimate the likely chance and degree of disturbance were that fistula to be laid open. This should then be put to the patient who can make an informed choice.
The internal opening feels like a small grain of sand or piece of rice and is slightly tender when pushed. As in all bodily systems, there is considerable redundancy: normal renal function with half of one kidney, the ability to resect more than half the liver or remove a lung; likewise with the anal sphincter quite a lot can be cut with little consequence. As a ball park figure, a consenting patient with normal bowel habit and without IBS could have 2 cm of cephalad anal sphincter left behind: two thirds would not notice continence disturbance, and one third would experience only inadvertent loss of flatus and occasional ‘skid marks’ on the underwear [8, 17]. In referral centres and with much experience of assessment that distance can be reduced to 1 cm and with some patients with a weekly bowel habit to 0.5 cm.
Any continence deficit noted at presentation is clearly also crucial. In a study of 84 patients, 50 of whom underwent fistulotomy and the rest permanent loose seton insertion, continence at referral was the only factor which predicted continence at discharge; 84 % of those continent at referral maintained full continence at discharge compared with 27 % of those with a continence impairment at referral (P < 0.001) [17]. There may also be a defect in the EAS distal to the internal opening from previous fistulotomy, leading to a ‘step-down’ or ‘keyhole’ deformity, which enables further fistulotomy to this level with impunity.
Understanding the Potential Anatomy
The key anatomical features proposed by Goodsall and Miles include location of the internal and external openings, the course of the primary tract and the presence of secondary extensions. Difficult cases include those with high tracts, secondary extensions and anterior fistulas in women, as well as those with inflammatory bowel disease.
There is no accepted definition of what constitutes a high fistula. In practice the key determination is not how much sphincter will be cut, but how much contracting sphincter will be left behind (just as in liver surgery, it is not how much liver is removed, but how much is left behind). Perhaps the word ‘complex’ is better than ‘high’, as it takes into account secondary extensions and the presence of inflammatory bowel disease or previous failed surgery.
We have previously defined a low fistula as one with a primary tract which is subcutaneous, intersphincteric or low transsphincteric (involving no more than the most distal 1 cm of external anal sphincter), and high fistulas as those with higher transsphincteric, suprasphincteric or extrasphincteric primary tracts [8]. By this definition, we would lay open many high fistulas as well as almost all low ones.
We would be cautious about anterior fistulas in women as the anterior sphincter can be very short.
Fistulotomy Technique
The surgeon needs a clear idea of bowel function and the patient’s consent before starting. Where there is doubt about fistula anatomy, MRI scanning using well-described techniques will help plan the approach and give a strong indication as to the likely site of the internal opening and of the presence of any secondary extensions. Mechanical bowel preparation and antibiotic prophylaxis are not normally employed. Some surgeons prefer an enema shortly before surgery.
Light general anaesthesia is preferred. Local anaesthesia, spinal/epidural anaesthesia and deep general anaesthesia with endotracheal intubation all end up with a paralysed external anal sphincter muscle which can make intra-operative judgment difficult. Under light general anaesthesia the anaesthesia can be lightened further leading to minor struggling or coughing, all of which contract the external anal sphincter and permit easy intra-operative identification of what length of external anal sphincter would be left behind were the fistula to be laid open.
Patient positioning is up to an individual surgeon’s preference. The authors prefer the lithotomy position with the ischial tuberosities on the end of the operating table and 120° of flexion at the hip (where possible). Illustrations used have adopted that orientation.
First, the area between the anus and the external opening is firmly palpated with a lubricated finger (Fig. 9.1). A superficial tract is easily felt, giving a clue as to where in the anus to find the internal opening. With a deep tract, nothing will be felt.
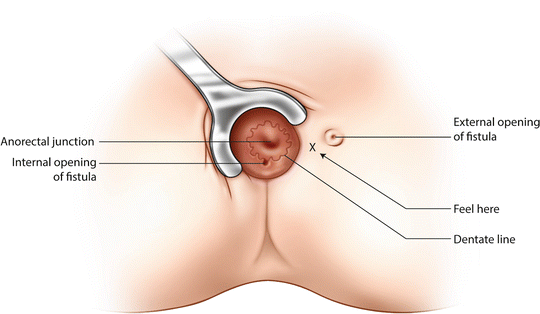

Fig. 9.1
Superficial palpation to detect the depth and direction of the fistula tract
The internal opening will usually be found at the dentate line and Goodsall’s rule usually applies. Injection of hydrogen peroxide (or a variety of other agents) may facilitate its identification, but is not fool proof, failing to demonstrate the internal opening when there is an epithelialised internal opening.
Supralevator induration takes experience to identify. The levator muscle should feel soft, like a fillet steak, but when there is sepsis in the vicinity it feels hard, like bone. The problem is that bone is expected when performing rectal examination—the sacrum, ischial tuberosities, coccyx and so on, so induration may be overlooked. It helps to compare the ‘softness/boniness’ of mirror images on the clock face of the anus (Fig. 9.2). Thus 4 o’clock with 8 o’clock, 1 o’clock with 11 o’clock. Prior MRI in such cases is extremely useful.
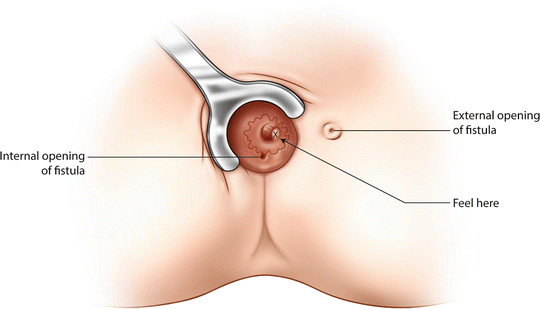

Fig. 9.2
Palpation of the supralevator area to detect induration indicative of a high primary tract or secondary extension
If supralevator induration is found having already palpated a more superficial tract, this heralds a secondary extension. On the other hand, having failed to palpate a superficial tract (Fig. 9.1), induration at the anorectal junction would be expected, denoting a high tract in the roof of the ischioanal fossa, as seen in most horseshoe fistulas.
The set of instruments needed are shown in the figure (Fig. 9.3). The partially curved Lockhart–Mummery fistula probe is perhaps the most useful. All probes must be handled gently, guided by knowledge already gained as to the site of the internal opening and a finger in the anus at that point. As with negotiating a bend at colonoscopy, slight pull-back while negotiating a bend prevents the tip impacting in the fistula wall and creating a false passage.
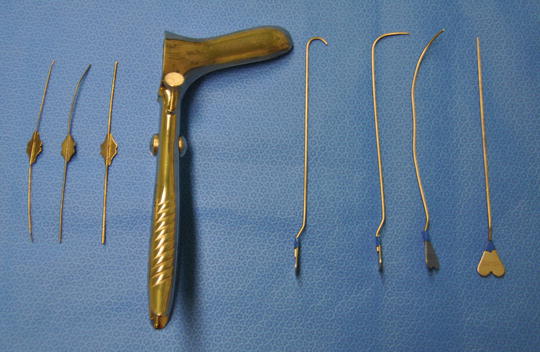

Fig. 9.3
Lacrimal probes (left), the Eisenhammer retractor and Lockhart–Mummery probes
Some fistula tracts are very narrow or tend towards an hourglass shape, such that the portion passing through the sphincter complex is not sufficiently wide to accept the probe (Fig. 9.4). In these cases hydrogen peroxide injected from the external opening may not exit the internal opening. A lacrimal probe will often negotiate such a tract. Once the probe has been passed through the tract and has entered the anal canal a further assessment can be made of the level of the fistula and the risk to function encountered if fistulotomy is performed. (Mostly, this judgment has already been made from the outpatient/office assessment. But to be able to make such a judgment in that setting requires a learnt ability to feel the internal opening in the conscious patient already described above. If not sure at this stage as to how much sphincter would be left behind were the fistula to be laid open, then the anaesthetist can be asked to lighten the anaesthesia, resulting in the anal sphincter contracting as outlined earlier. Alternatively, a loose seton may be placed and the patient re-examined when awake to determine the same.)
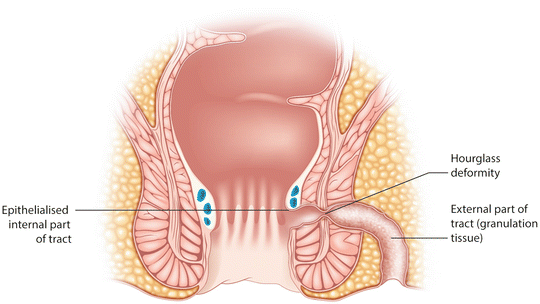

Fig. 9.4
Narrowing of the fistula tract with an hourglass deformity. A lachrymal probe may pass through if the Lockhart–Mummery probes do not
Some tracts cannot be negotiated with a fistula probe. The classic case is with a horseshoe fistula (Fig. 9.5). The strategy in these circumstances is depicted in Fig. 9.6.
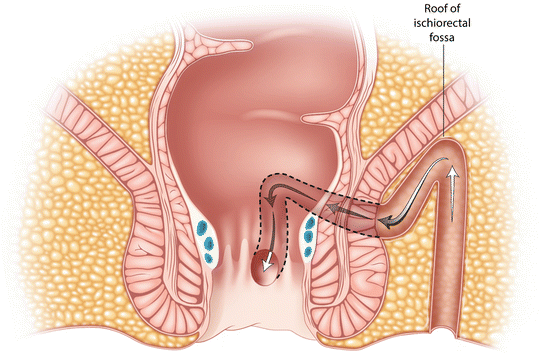
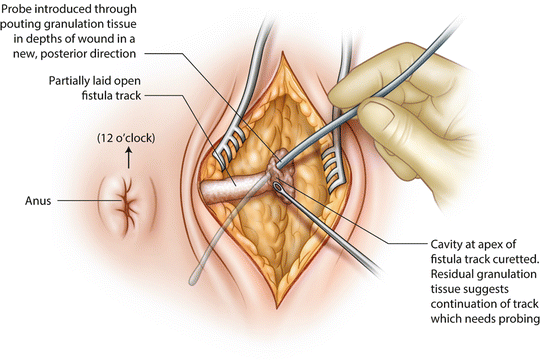

Fig. 9.5
Horseshoe fistulas may travel to the roof of the ischioanal fossa before running posteriorly towards the midline and then turning caudad before entering the anal canal

Fig. 9.6
A curved incision is made tracking the edge of the darkened anal pigment (which marks the outer boundary of the EAS) and this incision is deepened into fat. The tract is then laid open into this wound, avoiding damage to the EAS. Granulation tissue is curetted
Once the fistula has been negotiated a decision is taken whether or not to lay it open, mark it with a seton or adopt one of the other techniques covered in this book. The decision has already often been taken in the office/outpatient setting as described earlier, influenced by bowel habit, lack of IBS, patient’s wishes and so on. Suffice it to say that the important consideration is how much sphincter to leave behind, not how much to cut. To repeat: as a ball park figure, a consenting patient with normal bowel habit and without IBS could have 2 cm of cephalad anal sphincter left behind: two thirds would not notice continence disturbance, and one third would experience only inadvertent loss of flatus and occasional ‘skid marks’ on the underwear [8, 17]. In referral centres and with much experience of assessment that distance can be reduced to 1 cm and with some patients with a weekly bowel habit to 0.5 cm.
Once the fistula has been laid open, granulation tissue is curetted away meticulously. Any point where it is difficult to curette away granulation tissue heralds the possibility of a secondary extension and needs careful probing.
Where possible, the wound can be marsupialised (Fig. 9.7).
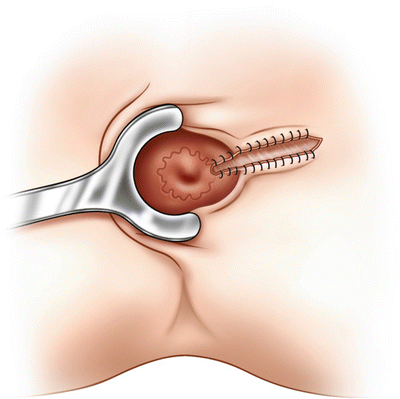

Fig. 9.7
Marsupialisation involves suturing the skin to the edge of the fistula tract with absorbable sutures to reduce wound size
Post-operative Care
The first 2 weeks with a large wound can be uncomfortable, but once lined with granulation tissue pain eases. There will be discharge and pus and blood and the patient will worry about infection, particularly when stools pass through the wound. Infection is actually rare. The wound can take 8 weeks or longer fully to heal, depending on its size and depth. Premature wound closure can lead to recurrence and can be prevented by regular wound digitation (which the patient or partner can be taught to do themselves once the wound is comfortable after the first fortnight). Sitz baths and various dressings are frequently used, but there is little evidence as to their efficacy.
Outcomes of Fistulotomy
The principle late complications are recurrence and impairment of continence, the latter not necessarily equating with dissatisfaction [6]. For example, Garcia-Aguilar reported in 2000 more dissatisfaction in patients suffering recurrence (61 %) than impairment of continence (24 %) [18].
Early complications relate to wound healing and discomfort. A randomised controlled trial of fistulotomy with and without marsupialisation from Thailand published in 2011 demonstrated reduced pethidine use in the marsupialised group but not improved healing [19]. Pescatori et al. showed the obvious, that marsupialised wounds were significantly smaller afterwards, and stayed smaller for 4 weeks [20]. They also found that bleeding was less but there was no difference in pain or septic complications. Ho et al. also found faster healing with marsupialisation (at a mean of 6 weeks compared to 10 weeks without) [21]. In a Cochrane review, the Ho and Pescatori studies were combined showing a small benefit in terms of recurrence and also, perhaps surprisingly, that incontinence was lower in the marsupialised group [1]. The review did not consider pain or wound healing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








