Fig. 1
Through the scope endoclips come in a variety or configuartions. They can be usefull for very acute closures
Several brands of clips are commercially available. These include the Quickclip (Olympus Corporation, Japan), the Resolution Clip (Boston Scientific, USA) and the Instinct and Triclip (Cook Medical Inc, USA). The first two have two prongs and the Triclip, as it name states, has three prongs and is the one that opens the widest (12 mm). All clips are currently made of stainless steel. Newer versions not commercially available yet (still in development) are titanium clips and even concepts for multi-firing devices [3].
Over-the-Scope Clips
The NOTES experience highlighted the need for secure, full-thickness closure of the GI tract [4]. One response from industry was the development of large clips that are attached to the end of the endoscope (“over the scope”) and are designed for robust, full-thickness closures and apposition of even relatively diseased tissues. There are currently two of this type of clips in the market that function very similarly. The over-the-scope clip (OTSC) (Ovesco, Tübingen, Germany) is a nickel–titanium alloy (Nitinol) clip that is highly elastic and has shape memory [5] (Fig. 2). It is designed to cover lesions from 9–11 mm (depending on the size of the clip). The clips are designed with different forms (atraumatic, sharp, or blunt edges) in order to be used in a range of scenarios from iatrogenic perforations to chronic fistulas. The OTSC has an ability to grasp more tissue than standard endoclips creating full thickness closure of a gastrointestinal wall defect, even in the presence of inflammation or induration.
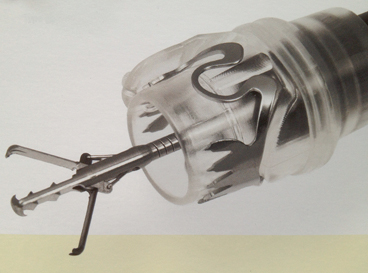

Fig. 2
Over the scope clips allow a more robust, full-thickness closure
The OTSC clip is delivered with an applicator cap mounted on the tip of the scope. A thread is attached to the clip and it is released by tightening the thread with a hand wheel. The caps come in different sizes (11, 12, and 14) to fit different endoscopes and in two different depths (3 and 6 mm) for grasping more or less tissue. Tissue capture is achieved by both robust tissue retraction and by sucking tissue into the cap. Ovesco has also produced two types of tissue retraction devices as standard endoscopic graspers are seldom robust enough to adequately pull tissue like this into the cap. These include an “anchor catheter” and a novel twin grasper (Fig. 3). The “anchor” is a long flexible catheter with three retractable pins that pierce the tissue in order to retract it into the cap and assist in the placement of the clip. The twin grasper is similar to a traditional grasper, but has the ability to open the two jaws of the grasper independently. This feature allows the endoscopist to grasp one side of the defect, hold it without releasing, and then grasp the opposite edge and approximate the tissue before releasing the clip. The OTSC received ‘Conformite European’ (CE) certification in Europe in 2009 and 510(k) clearance by the FDA in 2010 [1].


Fig. 3
Ovesco makes a clever double grasper to permit traction on both sides of a defect
The Padlock clip (Aponos Medical, Kingston, NH), is a similar design, but the firing mechanism is adjacent to the scope, and therefore, preserves use of the biopsy channel for instrumentation or suction/irrigation. It also comes in a variety of sizes and has a universal cap that fits on most types of endoscopes (Fig. 4). It too relies both on grasping the tissue and retracting and suction into the cap to achieve full-thickness closure. The Padlock received the FDA clearance in 2012, but is not currently cleared by a CE for sale in Europe.
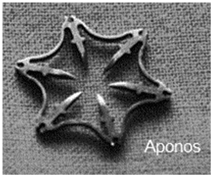

Fig. 4
The Padlock clip from Aponos, is another over the scope clip with full-thickness closure capabilities.
There have been multiple case reports and several small series that have documented the ability of the OTSC to close acute perforations, leaks, and fistulas [2, 5–12]. Animal studies have shown the superiority of the OTSC versus regular endoscopic clips in closing iatrogenic perforations [13]. The success is higher in GI perforation than for fistulas [1], but the preliminary results are promising. In order to facilitate the endoscopic closure of chronic perforations and fistulas, cauterization, debridement, and curettage of the margins should be performed. These techniques might improve healing around the edges of the perforation. Of note, is that once these clips are deployed, their characteristics make it extremely difficult to remove them without damaging the tissue between their jaws. The endoscopist must be certain that the clip is being placed in the right position before deployment. It has been suggested that exposure to cold water might aid in the removal of a misplaced nitinol OTSC; however, in our experience, we have found this to be unreliable. The development of a retrieval mechanism or technique would be a desirable feature in these types of clips. Risks reported with the use of the OTSC are perforation, bleeding, early detachment, mucosal lacerations, and although it has not been reported, luminal obstruction is a hypothetical complication.
Suturing Technologies
One of the strongest requests to industry from clinicians interested in NOTES was the ability to suture endoluminally. The result was a flurry of concepts designed that ranged from simple (T-tags [TAS, Ethicon, Blue Ash, OH]) (Figure 5) to more complex adaptations of laparoscopic suturing devices such as the Endostitch (Covidien, Norwalk, CN) Figure 6. There were even very complex flexible surgical platforms developed capable of bimanual suturing with standard surgical suture and techniques Fig. 7). Due to the costs of many of these technologies and the fact that they arrived during the onset of the global economic crises, only a few have succeeded in reaching the market.

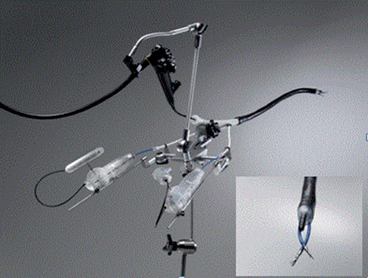

Fig. 5
T-tags were amoung the first endoscopic suturing devices

Fig. 6
Several devices were adaptations of existing laparoscopic devices like the Endostitch (Covidien, Ireland)

Fig. 7
An example of an endoscopic platform permitting laparoscopic like tissue interations – The DDES from Boston Scientific
G-Prox® (USGI, San Clemente, CA)
The G-prox is a suturing system that delivers a full-thickness pledgeted suture with a one-way cinch to tighten it after the placement (Fig. 8). The tissue retraction is best done using a helical tissue screw to allow robust closure. It has a unique way to insure safety when passing the needle full thickness through the GI wall (always an issue with full-thickness closures performed from inside the lumen). It does this by imbricating the wall of the GI tract into the lumen, so that the needle passes through the wall and back into the lumen, where the suture can be deployed under direct vision. The biggest downside to the G-Prox is that it is a 5 mm device, and therefore, cannot be used with standard endoscopes. USGI has a special multichannel 18 mm diameter flexible scope (transport) specially designed for the G-Prox (Fig. 9). The G-Prox has been used in several thousand cases to date, including fistula closures and closures of the gastric and colon wall after perforations or NOTES procedures. [14–19]
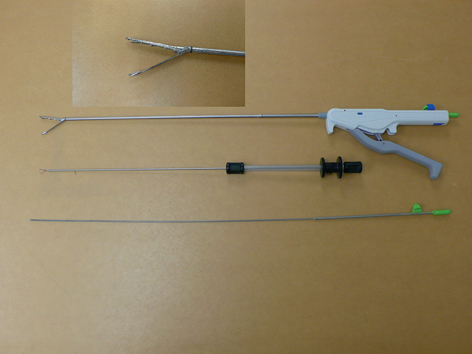


Fig. 8
The G-prox device from USGI medical

Fig. 9
The one drawback of the G-prox was the need to insert it via the Transport access device…
The Overstitch® Endoscopic Suturing System (Apollo Endosurgery, Austin, TX)
The Overstitch is a suturing device that is mounted onto a double-channel therapeutic endoscope and allows the placement of full thickness suture. It is compatible with the Olympus 2T160. To date, there is no compatibility with other brands of endoscopes. The device obtained FDA 510(k) clearance in 2008 [1]. The main part of the suturing device is a metallic cap placed on the tip of the endoscope. A needle driver placed within the cap holds and releases the actual needle attached to the suture. The needle serves as a T-tag when it is released. Available sutures include 2-0 and 3-0 polydioxanone (absorbable) and 2-0 and 3-0 polypropylene (nonabsorbable). The needle driver passes the needle from the mobile arm to the fixed arm piercing the tissue. The needle needs to return to the mobile arm of the needle driver before attempting to take another bite of tissue. After the suturing is finished, the needle is released and a cinching device consisting of a secondary tag slides through the suture and releases a cinch to hold the suture in place. The whole mechanism is maneuvered by a handle placed on the working channel of the endoscope (Fig. 10). The cinching device has its own release mechanism. A helix device can be used for an aggressive retraction. It consists of a helical tip that is screwed into the tissue and allows pulling it into the position for suturing. The overstitch is able to place running or interrupted sutures.


Fig. 10
The Overstitch suturing machine by Apollo Medical
A few reports exist to date showing the feasibility, safety, and durability of closures performed with the Overstitch. The first report in humans [20] presents a successful closure of a gastrocutaneous fistula after a percutaneous endoscopic gastrostomy (PEG) removal. The most common procedure the Overstitch is used for is bariatric revision surgery [21–26]. Thompson et al. reported on 25 patients who had weight regain after roux-en-Y gastric bypass due to pouch dilation or a patulous gastrojejunostomy [27]. At 1 year, patients lost an average of 11 kg with no adverse sequelae.
Flexible Endoscopic Platforms
Of the complex flexible endoscopic platforms designed for NOTES surgery and complex endoluminal procedures, only the Anubis scope (Storz, Tutlingen, Germany) is commercially available, and then, only on a limited release basis and only in Europe (Fig. 11). It is however, CE-marked and approved for human use and has been used in small case series for a variety of procedures. [28, 29]. The EndoSamurai (Olympus, Tokyo) and Direct Drive Endoscopic System (DDES, Boston Scientific, Natick, MA) are development projects that have been shelved by their respective companies due to the depressed economy and lack of a clearer picture of the clinical needs that they solve. These platforms offer the ability for two-handed tissue manipulation along with triangulation, so that it would seem perfect to replicate the ability to suture laparoscopically.
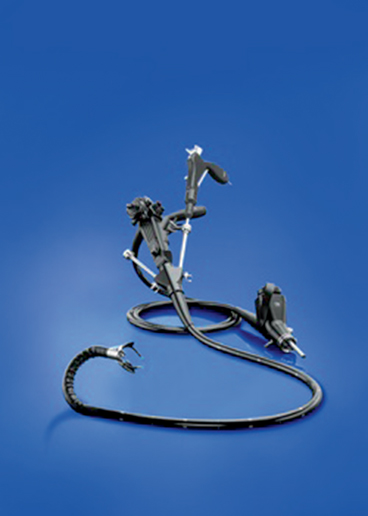

Fig. 11
The only commercially available advanced endoscopic platform – the Anubiscope by Storz
Covered Stents
Covered self expanding stents represent an important tool for dealing with all forms of breaches in the GI tract. Stents available in the market are self-expandable metal and plastic stents. Metal stents are composed of various materials like stainless steel, nitinol (nickel-titanium) with or without a silicon membrane, and elgiloy (cobalt, nickel and chromium). Nitinol stents are very flexible and elgiloy stents are corrosion resistant and capable of generating high radial force [30]. These metallic stents may be partially or fully covered by a plastic membrane [30]. Depending on the brand, they may have a distal and/or proximal flare to prevent migration [30] (Fig. 12). Plastic stents are approved for benign or malignant esophageal strictures. They have a woven polyester skeleton that is fully covered with a silicone membrane [30]. Although metallic stents are designed for malignant obstruction, they are widely used “off label” in the treatment of GI tract perforations. Noncovered metallic stents are equivalent to covered metallic stents in terms of technical success, clinical success, long-term patency, and survival rates for the palliative treatment of malignant obstruction in the digestive tract [31]. While stent migration or slippage is uncommon when they are used for tumor palliation, it is a major problem when covered stents are used to treat perforations as they often have no narrowing to hold the stent in position. Stent migration is also more common in covered stents, but noncovered stents are more prone to tissue ingrowths and obviously would be unsuitable for perforation treatment [31]. Stainless steel stents might also migrate more often than nitinol ones [31]. A variety of techniques to prevent migration have been described, but none is totally perfect. On a systematic review on benign esophageal rupture or anastomotic leaks, van Boeckel et al. [32] reported no difference in clinical success between fully covered, partially covered metallic, and plastic stents. Stent migration (25 % of patients) was more common in fully covered stents (plastic or metallic) than in partially covered stents. Tissue ingrowth was not significantly different. They recommended leaving the stents in place for approximately 7 weeks to achieve healing.
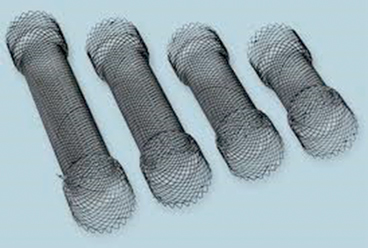

Fig. 12
stents come in many sizes and shapes
Miscellaneous Tools (Fibrin Glues, Atrial Septal Plugs, Acellular Matrix Fistula Plugs)
Sealants
The use of fibrin glue has been described as a fistula treatment for more than 20 years [33]. The theory behind its use is that the fibrinogen and thrombin in the fibrin glue combine in the presence of factor XIII and calcium chloride to form a fibrin clot. This fibrin plug acts as a physical barrier until healing begins [34]. Theoretically, it might also promote adhesions on the peritoneal side with migration of fibroblasts and subsequent collagen production. Small series report successful closing of gastrointestinal leaks after gastric bypass, sleeve gastrectomy, and biliopancreatic diversion with duodenal switch [35–37]. Although initially proposed for patients who are unfit to undergo a surgical procedure, it has been suggested that fibrin glue might be used alone or in combination with closure and/or stents for perforations in stable patients with no sepsis and who do not need immediate surgical treatment.
Acellular Matrix Fistula Plugs
SurgiSIS®, an acellular fibrogenic matrix biomaterial, is commonly used for hernia repair in the setting of intraperitoneal infection or where nonabsorbable synthetic materials are not desired (hiatal hernias). It is available in different sizes as tapered fistula plugs for treatment of fistula in ano and these have been used for the treatment of a variety of endoluminal fistulas as well (Fig. 13). Its use has been reported in gastrocutaneous fistula after Roux-en-Y gastric bypass [38, 39] with a success rate of 30 % after one application, 55 % after two applications, and 15 % after a third application for a total success rate of 80 %. A recent product introduced in the market is MatriStem® (Acell Incorporated, Columbia, Maryland). It is a porcine cellular structure that induces tissue growth. It is available as powder or as a sheet which can serve as a plug. The literature reports its use in the treatment of skin wounds [40]; however, initial experimental research is ongoing in the treatment of gastrointestinal perforations.


Fig. 13
An example of a cellular matrix fistula plug
Cardiac Septal Occlusion Devices
Off label use of the different cardiac septal plugs (Amplatzer Septal Occluder [AGA Medical Group, Plymouth, Minn] and the Cardio-SEAL septal repair implant [NMT Medical, Boston, Mass]) have been used for the closure of gastrointestinal fistulas and deliberate gastrostomies in NOTES [41]. These catheter-based devices consist of two self-expandable disks connected by a short waist (Fig. 14). The materials of the disks are woven nitinol with polyester inserts that are placed within the device to facilitate thrombosis and occlusion. When placed in the gastrointestinal wall, it allows healing of the mucosa over the disks. The real closure is achieved with the waist of the device. It functions by stenting the defect with its conjoint waist. The device achieves fixation and stability by stenting the defect and extra-stability is provided by the two atrial discs [42]. The device has the advantage of being easily repositioned or removed until it is completely released from the delivery wire, thereby allowing the removal in the event of malpositioning [42]. However, the disadvantage is that it can only be placed in a defect between two hollow cavities to allow placement of the discs in each cavity. A fistula defect with a long tract would not work for this device. Another problem is that it is a permanent foreign body that can fracture or migrate with time. Even more significant is the fact that these devices are expensive (several thousand dollars each).
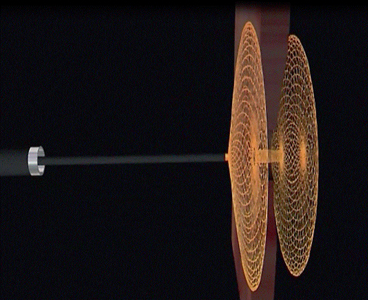

Fig. 14
a cardiac septal occluder device which has also been used to close GI tract defects
A case report of a gastrointestinal-related fistula include a gastrocolonic fistula that recurred 4 months after placing an Amplatzer device which was finally closed with a Cardio-seal implant with no evidence of recurrence for 18 months [41]. Other clinical experiences include the use of these devices for closure of tracheoesophageal fistulas [43–46]. The experience has not been completely satisfactory. Coppola [43] reported the enlargement of a tracheoesophageal fistula and migration of the device into the broncial tree 2 months after placing it with subsequent occlusion success after using partially covered stents and Kadlec [44] reported a dislodgment of the disc 12 days after placement with enlargement of the fistula finally requiring operative repair. Lee obtained better results although the follow-up was short (1 month) and Repici [45] obtained the best results closing a gastro-tracheal fistula after a gastric pull-up for esophageal cancer. Several attempts were made to close the fistula, including surgery, and the final attempt with the septal occlusion device was successful showing no recurrence in 8 months of follow-up.
Presentations and Indications for Treatment
Presenting symptoms vary according to the location of the leak, timing of the presentation for treatment, degree of body cavity contamination, and underlying patient disease. For acute perforations or leaks, there is usually a brief “golden hour,” where the patient is relatively stable, and where the immediate repair of the opening will affect a rapid cure. Acutely presenting leaks, if untreated, usually rapidly decompensate and can often progress to sepsis and cardiovascular collapse. For patients presenting in full septic shock, a trip to the operating room (OR) and emergent surgery is usually the best course, though endoscopic treatment that can often augment surgical exploration. An example would be a laparoscopic abscess drainage and debridement with concomitant placement of a covered stent. Imaging studies are usually not needed and can delay definitive treatment.
More chronic leaks can present with a wide spectrum of symptoms: once again, full-blown sepsis is a possibility, but also a more indolent presentation is possible. Sometimes, particularly with fistulous connections to other parts of the GI tract, the patient can be asymptomatic or have hard-to-diagnose conditions like low grade fevers, diarrhea, or weight regain after a bypass surgery. These patients require a high index of suspicion and a thorough workup. Contrast x-rays and/or computed tomography (CT) scans are usually the best mode of treatment though sometimes a diagnostic endoscopy is required for a definitive diagnosis. These patients are good candidates for a try at an endoscopic repair as surgery can be quite difficult and prone to complications.
Treatment Algorithms
Acute Perforation: A perforation occurring at the time of endoscopy should be repaired at the time of the occurrence or as soon as it is possible. This is best done by primary closure and it is one time that standard endoscopic clips often work well. If clip closure is not possible, a more robust closure technology can be used such as an OTSC or endoscopic suturing. If good closure can be achieved, no further adjuncts are needed though the patient should be kept nil per os (nothing by mouth, NPO) for a few days to allow healing. If doing well clinically, the patient can be started on a diet after 24–48 h.
Anastomotic Leak or Delayed Presentation Leak: Intermediate leaks, either ones that occur remote from surgery (from ischemic complications, ulcerations, etc.) or ones that were missed and presented later, require a more complex and multimodal approach for treatment. Primary closure is seldom possible or often requires adjunct treatments for success. A CT scan should usually be performed to document the extent of peritoneal, retroperitoneal, or thoracic contamination. Percutaneous drain placement should be established if indicated and possible. Endoscopy is the next step, with assessment of the breach. If the hole is found to have relatively fresh and pliable edges, primary closure can be considered. Standard clips are usually not feasible and either an OTSC or a suturing device is the better option.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








