Syndrome
Genes
Carney [35]
PRKA (protein kinase A)
Fanconi anemia [36]
About 15 different known genes, all involved in DNA repair
Familial adenomatous polyposis [53]
APC or less commonly MUTYH
8.3 Demographics
Overall, fibrolamellar carcinomas make up approximately 1 % of all hepatocellular carcinomas. Prior studies suggested a frequency of around 5 %, but more recent data from larger studies indicates a frequency closer to 1 % (Table 8.2). Fibrolamellar carcinomas have been reported from a wide variety of geographical locations, including most parts of the world. One study found no strong correlates between fibrolamellar carcinoma and white, black, Hispanic, or Asian ethnicities living in the USA [6]. However, many clinicians and scientists believe that fibrolamellar carcinomas are less common overall in Asian countries. There appears to be a small predilection in fibrolamellar carcinomas for males, but there is no evidence for a striking male dominance, as is seen in typical hepatocellular carcinoma.
Table 8.2
Representative studies of the prevalence of FLC in various populationsy
Nation | Prevalence (%) | Number of study cases (FLC/total) | Study time frame | Comment |
|---|---|---|---|---|
Sweden [43] | 0.4 | 2/532 | 1958–1979 | From Ostra Sjukhuset |
USA [6] | 0.4 | 191/46,731 | 2000–2010 | From SEER database |
Thailand [44] | 0.6 | 1/180 | 1991–1998 | From Songkhla |
USA [45] | 0.9 | 68/7896 | 1986–1999 | From SEER database |
Germany [46] | 1.1 | 13/1108 | 1998–2010 | From Mainz |
South Africa [47] | 3 | 9/274 | Pediatric cancer registry data; includes all pediatric cancers and not just HCC | |
Rochester, MN, USA [15] | 4 | 10/280 | 1987–1993 | From Mayo clinic |
USA [19] | 5 | 3/58 | 1966–1981 | From Ohio State |
Mexico [48] | 5.8 | 7/121 | 1987–2001 | From Mexico City |
Mexico [49] | 8.6 | 15/174 | 1990–2003 | From Mexico City |
United Kingdom [50] | 7 | 8/107 | Not stated | From London |
France [51] | 7.3 | 5/68 | From Villejuif; HCCs in non-cirrhotic livers | |
USA [16] | 8.9 | 41/477 | 1968 and 1995 | From Pittsburgh, PA; Patients undergoing liver transplant for HCC |
Canada [52] | 9 | 10/NS | 1982–1995 | From Toronto. Referrals to a tertiary care center for HCC with intent to treat from |
One of the most distinctive and well-recognized features of fibrolamellar carcinoma is its occurrence in younger individuals. In fact, 50 % of cases occur between the ages of 17 and 30 and 80 % occur between the ages of 10–35 [7]. Nonetheless, an important diagnostic point is that fibrolamellar carcinomas are not the most frequent form of liver cancer in children and young adults. Instead, typical hepatocellular carcinomas are still the most common and account for 60–80 % of cases [1, 8–10]. Thus, young age is not a finding on which a diagnosis of fibrolamellar carcinoma can be based.
8.4 Clinical Findings
Fibrolamellar carcinoma most often presents with vague, nonspecific abdominal pain, weight loss, and malaise. However, there is a wide variety of less common findings at presentation. Of these, one recurrent theme is biliary obstruction secondary to direct tumor growth into the biliary tree or to compression by metastatic deposits in hilar lymph nodes. Gynecomastia in males is also a rare but distinctive finding at presentation.
8.5 Serum Findings
Serum alpha-fetoprotein (AFP) levels are normal in essentially all cases and an elevated serum AFP level makes the diagnosis of fibrolamellar carcinoma unlikely. There are some reports of fibrolamellar carcinomas with significantly elevated serum AFP levels, and it is possible that this might occur in rare cases, but overall it seems likely that most of these cases are misdiagnosed.
A number of serum proteins are elevated in individuals who have fibrolamellar carcinoma, but none are sufficiently sensitive or specific to be widely used clinically. These proteins include transcobalamin I, transcobalamin 2, fibrinogen, and neurotensin. In addition, PIVKA-II (protein induced by vitamin K absence/antagonist-II) is commonly elevated in both typical hepatocellular carcinomas and in fibrolamellar carcinoma. While these serum findings are not useful for diagnosis, they can be useful in individual cases to monitor for response to tumor therapy and to monitor for tumor recurrence.
8.6 Prognostic Factors and Tumor Spread
Prognostic factors are shown in Table 8.3. After resection of primary disease, approximately 55 % of cases will recur within the first 5 years [11]. Recurrent disease is intrahepatic in about 40 % of cases, involves extra-abdominal organs such as the lungs in about 40 % of cases, and presents with peritoneal and/or lymph node disease in about 20 % of cases [11].
Table 8.3
Prognostic factors in fibrolamellar carcinoma
Prognostic factor | Comment and representative reference |
|---|---|
Gender | Females have better prognosis [45] |
Age | |
Absence of elevated liver enzymes | Better prognosis [49] |
Absence of large vessel invasion or thrombosis | Better prognosis [49] |
Lymph node disease at presentation | |
Metastatic disease at presentation | Worse prognosis [16] |
Stage | |
Vascular invasion | Worse prognosis [16] |
Lymph node metastases are identified at presentation in approximately two thirds of fibrolamellar carcinoma cases [12–15]. In fact, lymph node metastases at the time of presentation are more common in fibrolamellar carcinoma than in typical hepatocellular carcinoma [3]. The involved lymph nodes are commonly regional nodes [16–18], including celiac [16, 18], gastric [19], and para-aortic lymph nodes [16, 19].
Direct extension outside of the liver is also common at presentation. In one study, 42 % of fibrolamellar carcinomas extended into the adjacent fat planes by imaging studies [13]. Direct extension into adjacent organs can also be seen, including the stomach [19], diaphragm [13], and pancreas [13, 20]. A subset of cases can also demonstrate widespread peritoneal disease at presentation [13–16, 21–23]. Other cases tend to spread by isolated or a small number of metastases, a pattern that can often be treated with surgery.
8.7 Gross Findings
Overall, fibrolamellar carcinomas tend to be more common in the left lobe of the liver, but frequently involve both lobes. In 80–90 % of cases a single large tumor is present. In cases with multiple tumor masses, there tends to be one dominant mass. Fibrolamellar carcinomas tend to be large at the time of resection, commonly measuring 10 cm or more in greatest dimension. Fibrolamellar carcinomas range in color from yellow to pale tan (Fig. 8.1) and range in consistency from soft to firm. The tumors can feel gritty when microcalcifications are prominent. In cases with prominent pseudoglands, the tumor can have areas that are grossly tacky and mucinous. A central scar is found in about two thirds of cases. Occasionally, the tumors may contain small foci of necrosis or areas of intratumoral hemorrhage. Gross vascular invasion is seen in up to 25 % of cases [16]. The background liver is non-cirrhotic, but a rim of inflamed and fibrotic tissue is often present at the interface of the fibrolamellar carcinoma with the non-tumor parenchyma. Sections from this area are helpful and important for looking for angiolymphatic invasion, but are not useful for examining the background liver for underlying liver disease.
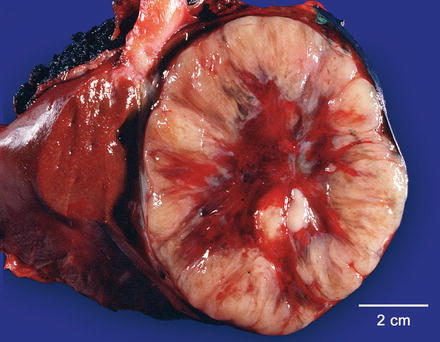

Fig. 8.1
Fibrolamellar carcinoma, gross. The tumor is yellow with a central area of fibrosis
8.8 Histologic Findings
Tumor cells are large and polygonal, with abundant eosinophilic cytoplasm that is rich in mitochondria and lysosomes (Fig. 8.2). The nuclei often have vesiculated chromatin and large nucleoli (Fig. 8.3). These distinctive cytological findings, when combined with the intratumoral fibrosis, are the defining H&E features of fibrolamellar carcinoma.
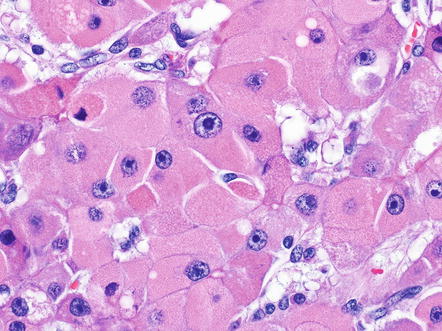
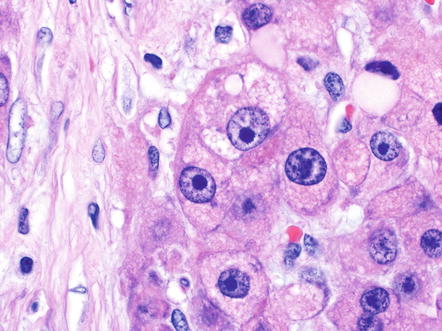

Fig. 8.2
Fibrolamellar carcinoma, cytoplasm. Fibrolamellar carcinomas have large eosinophilic tumor cells

Fig. 8.3
Fibrolamellar carcinoma, nucleoli. Many of the tumor cells in fibrolamellar carcinoma have prominent nucleoli. The nucleoli can be either eosinophilic or basophilic, depending on the staining preparation
There are a variety of other histological findings that are common in fibrolamellar carcinoma, but they are neither necessary nor specific for the diagnosis. The most distinctive are pale bodies. Pale bodies are round amphophilic inclusions found in approximately 50 % of cases (Fig. 8.4). Pale bodies do not show any clear spatial relationships with fibrosis, the center or periphery of the tumor, or tumor vasculature. They are immunoreactive for a number of different proteins, including vimentin, but the principal protein component is not known. Their cause is also not known, but presumably reflects a defect in protein folding or protein secretion. Hyaline bodies can also be found in 20–30 % of cases, but can be more subtle histologically than pale bodies, and their true frequency is not clear. Hyaline bodies are eosinophilic cytoplasmic inclusions of unclear etiology and composition (Fig. 8.5). Similar to pale bodies, there does not appear to be any strong spatial correlates and hyaline bodies appear to be somewhat randomly found in tumors. Pale bodies and hyaline bodies can be found in the same tumor, but are generally not found in the same areas within a tumor.
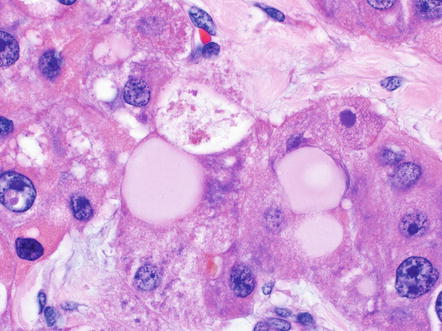
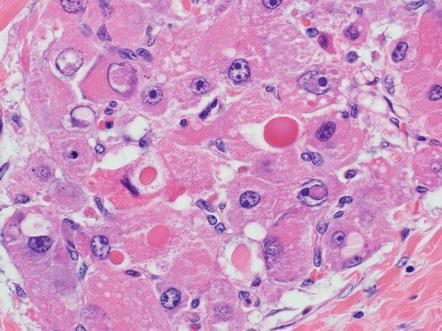

Fig. 8.4
Fibrolamellar carcinoma, pale bodies. Pale bodies are large amphophilic cytoplasmic inclusions. They are common in fibrolamellar carcinoma, but not specific

Fig. 8.5
Fibrolamellar carcinoma, hyaline bodies. Hyaline bodies can range considerably in size, but are dense eosinophilic inclusions, found mostly in the cell cytoplasm, but in some cases appear to be extracellular
Approximately 10 % of fibrolamellar carcinomas can have gland-like structures, or pseudoglands (Fig. 8.6). The pseudoglands are ovoid cystic structures lined by tumor cells, have a wide range of sizes, and in some cases appear to arise from dilated canalicular like structures. The secretions within the pseudoglands are mucicarmine positive in about half the cases and are Alcian blue positive in most cases. Because of the mucicarmine positivity, some have suggested these are true glands and not pseudoglands, leading to diagnoses of combined fibrolamellar carcinoma and cholangiocarcinoma. Despite the mucicarmine staining, the data at this point suggests they should be classified as ordinary fibrolamellar carcinomas and not as combined tumors.
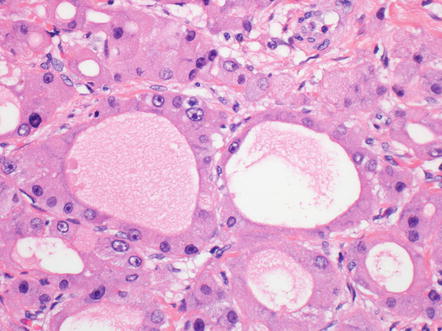

Fig. 8.6
Fibrolamellar carcinoma, pseudoglands. Sometimes, variation in size can be seen, with the smallest appearing to arise from dilated canalicular like structures
Metastases to lymph nodes and other organs typically look like the primary tumor, but in some cases metastatic disease can have a prominent pseudoglands component (Fig. 8.7). Since these pseudoglands can produce mucin, they can be mistaken for a cholangiocarcinoma. However, a history of fibrolamellar carcinoma is often present and will aid in the proper diagnosis. In other cases, immunostains can be used to differentiate fibrolamellar carcinoma from adenocarcinoma, as fibrolamellar carcinomas will be positive for HepPar1, arginase, CK7, and CD68.
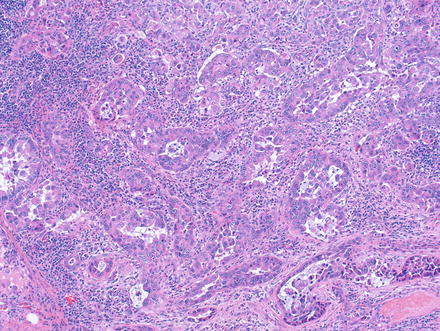

Fig. 8.7
Fibrolamellar carcinoma, metastatic to lymph node. The presence of pseudoglands in this case mimics cholangiocarcinoma
Other common tumor findings include microcalcifications. The calcifications can be dystrophic, featuring irregular deposits in areas of remote necrosis, fibrosis, or the walls of larger vessels. In other cases, the microcalcifications appear to represent calcification of single tumor cells (Fig. 8.8). Mild macrovesicular steatosis can occasionally be seen, even in cases without fat in the background liver (Fig. 8.9). Sometimes small pseudoglands can be similar in size to fat droplets and the two can appear similar at low power, but higher power examination readily separates the two findings. Tumor cholestasis is also common, with bile located within the cytoplasm of tumor cells or within canalicular like structures between tumor cells (Fig. 8.10). This frequent cholestasis is commonly associated with copper accumulation within tumor cells. However, copper accumulation is also found in ordinary hepatocellular carcinomas that are cholestatic and thus is not a specific finding. Finally, occasional benign isolated bile ducts can be found entrapped within the growing front of the tumor (Fig. 8.11).
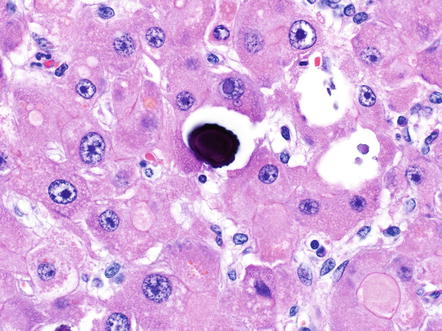
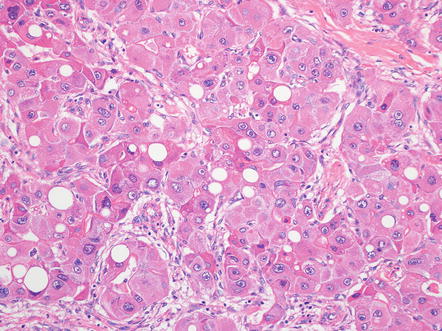
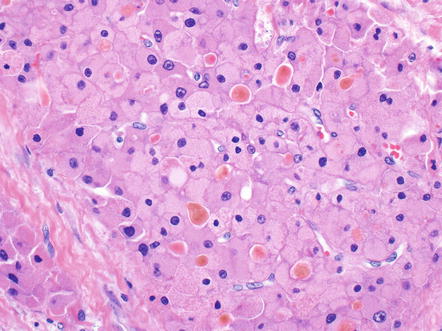
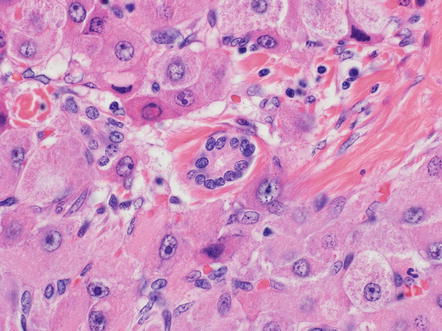

Fig. 8.8
Fibrolamellar carcinoma, microcalcifications. This microcalcification appears to represent a single dead, calcified cell

Fig. 8.9
Fibrolamellar carcinoma, fatty change. Mild macrovesicular steatosis is seen

Fig. 8.10
Fibrolamellar carcinoma, cholestasis. Canalicular cholestasis is present in this tumor

Fig. 8.11
Fibrolamellar carcinoma, entrapped bile duct. An entrapped benign bile duct is seen in the center of this image
Vascular invasion is present in 40–50 % of fibrolamellar carcinomas on histological analysis [8, 14, 16]. Vascular spread into the portal veins of nearby portal tracts is a common pattern of angiolymphatic invasion. The best place to identify vascular invasion is sections taken at the tumor–nontumor interface.
Perineural invasion can be seen when tumors involve the hilum of the liver (Fig. 8.12). In addition, tumor spread into the soft tissue of the liver hilum is a common finding and one that does not readily fit into current tumor staging systems. Nonetheless, both perineural invasion and invasion of the liver hilum soft tissue can be included in pathology reports and presumably indicate a worse prognosis.
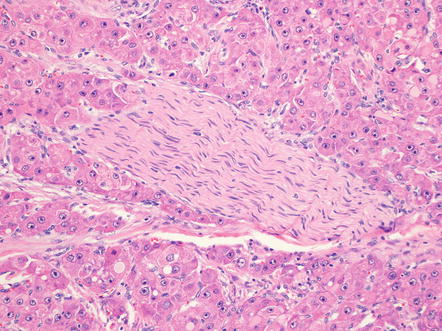

Fig. 8.12
Fibrolamellar carcinoma, perineural invasion. Perineural invasion is seen most commonly in tumors involving the liver hilum
8.8.1 Grading
Fibrolamellar carcinomas are histologically homogenous and lack the range of differentiation, from well differentiated to poorly differentiated, that is seen in ordinary hepatocellular carcinomas. Nonetheless, tumor templates demand a tumor grade; in that context, essentially all primary fibrolamellar carcinomas are moderately differentiated, as well as most recurrent and metastatic tumors. In fact, poorly differentiated cytology is sufficiently unusual for fibrolamellar carcinomas that any poorly differentiated tumor should be carefully examined before making a diagnosis of fibrolamellar carcinoma.
8.8.2 Intratumoral Fibrosis
Intratumoral fibrosis is a characteristic component of fibrolamellar carcinoma (Fig. 8.13) and is found in essentially all primary tumors and in many metastatic tumors. Nonetheless, the amount of intratumoral fibrosis varies and there often can be areas with a more solid growth pattern that lack intratumoral fibrosis, but retain the otherwise typical cytological findings (Fig. 8.14). These cases should be diagnosed as fibrolamellar carcinoma and not as a combined hepatocellular carcinoma and fibrolamellar carcinoma. Also of note, the fibrosis will be deposited in parallel, or “lamellar,” bands in many cases of fibrolamellar carcinoma, but some cases will have more irregular patterns of intratumoral fibrosis. The fibrosis in about two third of cases coalesces into a larger central scar.
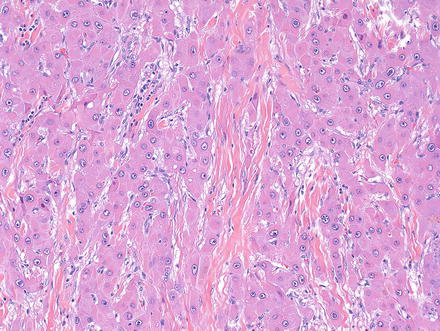
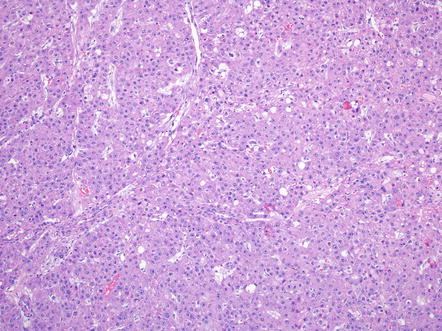

Fig. 8.13
Fibrolamellar carcinoma, lamellar intratumoral fibrosis

Fig. 8.14
Fibrolamellar carcinoma, solid growth. In this tumor, large areas had limited or no intratumoral fibrosis
8.9 Immunohistochemistry
Fibrolamellar carcinomas show unequivocal hepatocellular differentiation by immunohistochemistry, but also show expression of proteins more commonly associated with biliary differentiation. The frequency of staining with commonly used diagnostic immunostains is shown in Table 8.4. Neuroendocrine features have been noted in some fibrolamellar carcinomas by several authors [24–26]. Most fibrolamellar carcinomas are negative for chromogranin and synaptophysin by immunohistochemistry [27, 28], but occasional cases are chromogranin positive [17].
Table 8.4
Immunostains in fibrolamellar carcinoma
Immunostain | Frequency of positive staining (%) | Comment and representative reference |
|---|---|---|
Markers of hepatic differentiation | ||
HepPar | 100 | |
Arginase 1 | 100 | |
Glypican-3 | 20–60 | |
Albumin-ISH | 100 | [27] |
Polyclonal CEA
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||





