Fig. 11.1
Schematic of classic coagulation cascade
During the provisional matrix that forms in the wound during early healing, fibrin becomes coated with vitronectin from the serum and fibronectin derived from both serum and aggregating platelets. Fibronectins are a class of glycoproteins that facilitate the attachment of migrating fibroblasts as well as other cell types to the fibrin lattice. Because of its influence on cellular attachment, fibronectin is a key modulator of the migration of various cell types in the wound. Additionally, the fibrin-fibronectin lattice binds various cytokines released at the time of injury and serves as a reservoir for these factors in the later stages of healing [19, 20]. The theory behind the treatment of fistulae with fibrin sealant is twofold. First, occlusion of the fistula tract with sealant immediately halts the ongoing contamination of the tract with stool, mucus, blood, and pus. Second, the proteins contained within the sealant stimulate native tissue in-growth and provide biologic scaffolding for the wound-healing process. The sealant is degraded as the fibrotic reaction progresses, and ultimately the sealant is entirely replaced by native tissues. Thus, no foreign body persists and the tract simply scars closed [21]. Fibrin gluing of anal fistulas is simple and repeatable. These factors make it a highly desirable treatment option. The use of fibrin sealant has grown in popularity over the last one and a half decades, although its appeal may be waning because of the variable results published over time.
History
Fibrin tissue adhesive was first used successfully as a hemostatic agent in the early 1900s [19]. The efficacy of fibrin sealant was markedly improved through the addition of bovine thrombin to fibrinogen in 1944 [22]. Commercial plasma fractionation methods in the 1970s generated highly concentrated fibrinogen preparations that were made available in Europe in the late 1970s. Unfortunately, pooled fibrinogen concentrates were associated with an increased risk of viral transmission, especially hepatitis B and hepatitis C and later HIV. This led to license revocation in the United States by the Food and Drug Administration in 1978. Two decades later in 1998, the Food and Drug Administration relicensed the commercial preparation of fibrin sealant.
In the United States prior to 1998 fibrinogen was obtained primarily through autologous donation and “home-made” preparations. Implementation of viral inactivation procedures has made the use of commercial sealants quite safe and popular now and the preferred source of fibrin due to its ease of use and quick preparation, as evidenced by abundant clinical literature from throughout the world.
Autologous Fibrin Glue
The use of an autologous source to prepare fibrin glue minimizes the risk of disease transmission and provides a safe and simple method to treat anorectal fistulas. Abel et al. [23] published their results on the use of autologous fibrin glue in the treatment of rectovaginal and complex fistulas in ten patients and reported an overall success rate of 60 %. The authors combined autologous fibrinogen in cryoprecipitate (AFTA-C) with reconstituted bovine thrombin, thereby reproducing the final stage of the coagulation cascade. This process was reported to recover approximately 20–40 % of the fibrinogen in a unit of plasma that in total yielded approximately 10–35 mg/mL of fibrinogen concentrate. The fibrinogen concentrate is then combined with reconstituted thrombin (1,000 U/mL). Unfortunately, the process of autologous fibrinogen preparation through cryoprecipitation (AFTA-C) in the study by Abel et al. [23] took greater than 24 h to manufacture and required donation of a unit of blood. In addition, patients in the study by Abel et al. [23] underwent outpatient bowel preparation, received preoperative parenteral antibiotics, and stayed in the hospital taking nothing by mouth for 2 days postoperatively.
Autologous fibrin tissue adhesive made from a patient’s own blood and based on ammonium sulfate precipitation (AFTA-A) is another method of producing autologous fibrin tissue adhesive. This tissue adhesive is biodegradable, is without side effects, and minimizes the risk of viral transmission. However, the bonding power of AFTA-A is significantly less than commercially produced fibrin tissue adhesives, hence limiting its effectiveness in cases where bonding power is essential such as in anorectal fistulas.
Another alternative method of producing autologous fibrin tissue adhesive uses a combination of ethanol and freezing to precipitate fibrinogen (AFTA-E). This method produces a biodegradable, autologous, and superior bonding power product than AFTA-A. AFTA-E is a third-generation autologous fibrin tissue adhesive developed after the first-generation (AFTA-C) and second-generation (AFTA-A) adhesives. The technical aspects of preparation of AFTA-E have been reported elsewhere [24]. Component one of AFTA-E is manufactured from 100 mL of a patient’s blood. The fibrinogen is obtained via ethanol precipitation. Component two of the adhesive is prepared by combining a calcium chloride solution with thrombin and aminocaproic acid. The final thrombin concentration is 450 U/mL and the total preparation time for ATFA-E is 60 min. The results reported by Cintron et al. [25] using autologous fibrin glue parallel those of prior generation tissue adhesives [23]; however, several important differences should be pointed out. The use of a third-generation autologous fibrin tissue adhesive (AFTA-E) allows the manufacture of fibrin sealant within 1 h of a scheduled operation in contrast to 24 h. In addition, the fibrinolytic inhibitor, aminocaproic acid, keeps AFTA-E present in vivo for over 40 days at the reported concentration [26]. Furthermore, a sufficient quantity of fibrinogen (3–4 mL) is precipitated from 100 mL of blood, which when combined with an equal volume of bovine thrombin adequately fills the fistula tracts. Thus, large blood donations are avoided. All procedures were done on an ambulatory basis, and bowel preparation, parenteral antibiotics, and fistula tract decontamination were not performed unlike the studies by Abel et al. [23] and Hjortrup et al. [27], respectively.
Commercial Fibrin Sealant
By the 1970s, highly concentrated fibrinogen became widely available, as did Factor XIII and aprotinin, which served to stabilize the fibrin clot. In 1978, however, the United States Food and Drug Administration (FDA) prohibited the use of fibrinogen concentrates derived from pooled donors because of the risk of viral transmission of hepatitis (and later HIV). As a result, surgeons in the United States were left to use single-donor fibrinogen products and bovine aprotinin. By 1998, donor screening, reliable testing methods, and viral deactivation techniques made pooled fibrinogen products safe again. The FDA subsequently approved the use of commercially produced products for patients. Since that time, the use of fibrin sealant has been described for nearly every organ system. The combination of the two components of fibrin sealant reproduces the final stage of the native clotting cascade. The two essential components are fibrinogen and thrombin. The thrombin converts the fibrinogen into active fibrin. One of the commercial products most widely used is Tisseel® VH fibrin sealant (Baxter Healthcare, Deerfield, IL). The sealant is available as a two-component system. One component contains a solution of fibrinogen, Factor XIII, and bovine aprotinin. The second contains thrombin and calcium, which acts as a cofactor. The two components are maintained in separate syringes until a specially designed dual syringe applicator (Duploject®, Baxter Healthcare) (Fig. 11.2) delivers the products to the surgical site. The two components remain separated until they are mixed at the tip of the applicator device. The fibrin clot begins to organize within seconds of the two components mixing. As with autologous fibrin glue the fibrin matrix contained within the clot also serves as scaffolding for tissue in-growth into the healing wound. The fibrin as well as the fibronectin and glycoproteins that migrate into the clot stimulate activate fibroblasts, collagen deposition, re-epithelialization, and neovascularization of the wound. In this way the sealant facilitates the wound healing process. The body’s native plasminogen system will destabilize the clot, and within 2 weeks, the entire synthetic clot is destabilized and replaced by host tissues [19, 20].
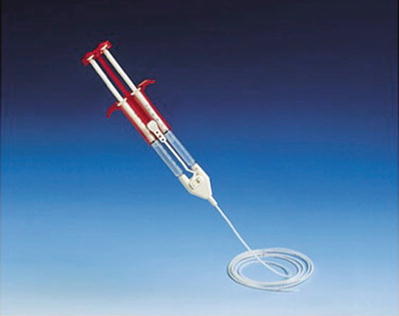

Fig. 11.2
Duploject® catheter system
Fibrin Sealant as a Carrier or Delivery Vehicle
Fibrin sealant has also been utilized to deliver cytokines, biomaterials, and most recently stem cells to the site of anal fistulas [28–30]. Singer et al. [29] reported on the use of fibrin sealant as a delivery vehicle for transforming growth factor beta (TGF-β) in an acute and chronic wound model in rats. Transforming growth factor is known to stimulate the inflammatory cascade and the wound healing process. They concluded that although fibrin sealant was an adequate delivery vehicle for TGF-β, unfortunately, it did not result in any significant changes in the healing of acute or chronic wounds in rats. Hammond et al. [28] assessed the safety, feasibility, and efficacy of cross-linked collagen in two different formats to heal anal fistulae. At operation patients were randomized to receive a solid collagen implant vs. collagen fibers suspended in fibrin glue. At the end of 29 months 80 % of the patients who underwent collagen-fibrin glue treatment were healed compared to 54 % who received the collagen implant alone. Garcia-Olmo and colleagues [31] reported on a randomized controlled multicenter Phase II study looking at fibrin glue vs. fibrin glue with adipose-derived stem cells in the treatment of 49 patients with complex perianal fistulas. After a 1-year follow-up there was a 16 % success rate in patients receiving fibrin glue alone compared to 71 % for patients who received fibrin glue in combination with adipose-derived stem cells. Herreros et al. [30] subsequently reported their results from a multicenter, randomized, single blind phase III trial utilizing autologous-expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas. Patients underwent surgical closure of the internal opening and then were randomized to receive either stem cells alone, stem cells with fibrin glue, or fibrin glue alone. The authors concluded that healing rates of approximately 40 % at 6 months were equivalent to fibrin glue alone and that when the three groups were compared no statistically significant differences were found. The utilization of fibrin sealant for these applications is still in its infancy and continues to evolve.
Technique
Although the operative procedure for fibrin glue injection of anal fistulas in the United States was performed with autologous fibrin sealant prior to 1998, most surgeons now utilize commercially prepared fibrin sealant when gluing anorectal fistulas. The reasons for this are multiple, including high fibrinogen concentrations with commercially prepared products, uniform production, advanced viral inactivation techniques, easy and quick preparation, no need for patient blood donation, greater quantities easily available, and consistent high bonding power. Operative procedures are typically performed as an outpatient. Preoperative mechanical bowel preparation is not required, other than an enema on the morning of surgery to evacuate the distal rectum. Oral and/or intravenous antibiotics are not necessary for this procedure. Patient positioning is at the discretion of the surgeon, provided that the primary and secondary openings of the fistula are easily accessible. The secondary or external opening is easily identified. Location of the primary or internal opening is essential in order to improve the success of the procedure. Occasionally hydrogen peroxide is utilized in order to inject the fistula tract in order to locate the primary opening. The tract should then be gently debrided without undue dilatation of the tract. Either an unfolded gauze sponge, a silk suture with a series of knots, a small curette, or a cytology brush works well (Fig. 11.3). Aggressive curettage or debridement should be avoided so as not to dilate the fistula tract. Dilation of the tract can lead to a greater quantity of sealant required to fill the fistula and to a higher risk of fibrin clot extrusion from the tract. After debridement the tract should be irrigated with saline or hydrogen peroxide to further cleanse the tract. Iodine irrigation of the tract should be avoided because iodine solutions can destabilize the fibrin clot. The fibrin sealant is prepared according to the manufacturer’s instructions. A dual syringe applicator and dual lumen catheter is utilized containing the two components, which will mix together at the tip when injected. A variety of delivery systems are available. The author prefers a long, flexible catheter tip as seen in Fig. 11.2. Other delivery systems are available including malleable dual lumen catheters (Fig. 11.4). The dual lumen catheter is passed through the entirety of the fistula tract, at least up to the internal or primary opening and in most cases preferably through the internal opening. The catheter tip is first placed into the external orifice, through the tract, and into the anal canal towards the primary opening. This is usually accomplished by placing a tie/seton through the tract initially, which can then be secured to the catheter. The tie is then used to drag the dual lumen catheter with it and into the tract towards the primary opening (Fig. 11.5). The sealant is slowly injected at the internal opening and allowed to set (Fig. 11.6a). Once the clot stabilizes at the primary opening, the catheter is slowly withdrawn through the tract as sealant is being injected, thus obliterating the entire tract (Fig. 11.6b). The clot is allowed to solidify for 5–10 min. Figure 11.7a–c graphically demonstrates the injection process. The external orifice is then dressed with a non-adherent dressing. Patients are discharged home on the day of surgery, as there is minimal or no postoperative pain. Patients are instructed to avoid strenuous activity and are placed on a bowel regimen for approximately 2 weeks. Additionally, patients are instructed not to take Sitz or tub baths for 2 weeks, so as not to prematurely disrupt the fibrin clot. Showering is permitted. Complete obliteration of the tract and any of its side branches with sealant is the critical feature of the procedure. If an abscess is identified at the time of examination, it should be drained and a seton placed, and fibrin gluing deferred for a later date.
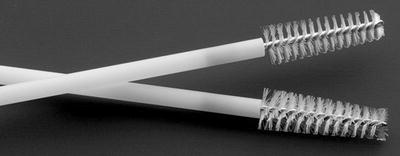
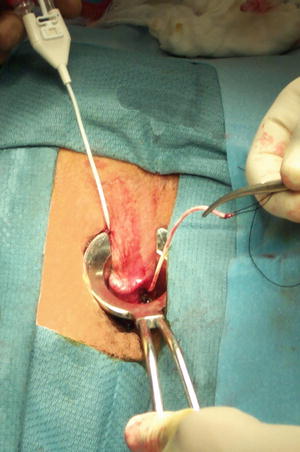
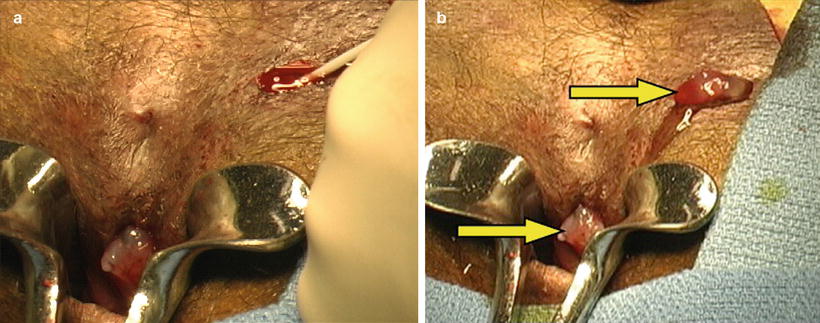
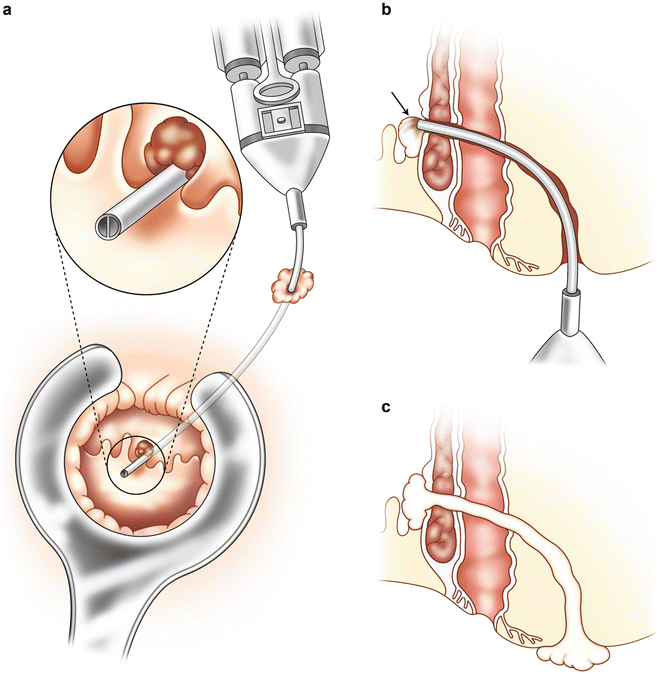

Fig. 11.3
Cytology brush used to debride fistula tract

Fig. 11.4
First-generation Micromedics® malleable catheter system. With permission © Micromedics Inc., St. Paul, MN

Fig. 11.5
Seton used to drag dual lumen flexible catheter through fistula tract

Fig. 11.6
(a) Dual lumen catheter trimmed and injection commenced occluding primary opening. (b) Completed injection demonstrating fibrin plugs present by arrows at primary and secondary fistula orifices (With permission Singer et al. [40])

Fig. 11.7
(a) Dual lumen catheter system in place ready for fibrin sealant injection. (b) Fibrin sealant injection commenced with fibrin plug present at primary opening. (c) Fibrin sealant injection completed with entire tract sealed and plugs present at the primary and secondary openings (With permission Singer et al. [40])
Complications Associated with Fibrin Sealant
One of the most common complications associated with the use of fibrin sealant for anorectal fistulas is the development of infection typically at the site of the external or secondary opening. This is reported in approximately 0–10 % of patients. It is important not to suture close the secondary opening at the time of gluing as this can lead to an increased incidence of infection. Other complications or side effects may be secondary to the components that constitute the product itself. These include but are not limited to hypersensitivity or allergic anaphylactoid reactions (bradycardia, tachycardia, hypotension, flushing, bronchospasm, wheezing, dyspnea, nausea, urticaria, angioedema, pruritus, erythema, paresthesias) as well as infectious risks. Anaphylactic reactions to the antifibrinolytic protein aprotinin have been reported especially in patients who have had prior exposure to aprotinin [32, 33]. Additionally, as the commercial sealants are manufactured from human plasma, there is always the risk that the plasma may contain infectious agents such as known viruses (parvovirus), emerging viruses, or other pathogens that can potentially transmit disease including Creutzfeldt–Jakob disease (CJD) that are not eliminated by current inactivation procedures [33].
In autologous preparations or in preparations in which bovine thrombin is used, there have been some reports regarding excessive bleeding following the use of bovine thrombin particularly after reexposure to thrombin [34, 35]. Some patients have been reported to develop acquired coagulation factor inhibitors in response to bovine thrombin exposure. This does not seem to be the case when patients are reexposed to recombinant human thrombin which is utilized with greater frequency today [36]. The antibodies to bovine Factor V have been shown to elicit cross-reactivity with human Factor V, which potentially can decrease the amount of Factor V available, with subsequent inhibition of the clotting cascade [37]. This reaction is minimized via lower thrombin concentrations and through the use of Factor V-depleted bovine thrombin preparations [38].
Literature Review
Over the past one and a half decades there have been an increased amount of publications on the topic of fibrin glue in the management of anal fistulas that corresponds to the period after the FDA approved commercial sealants for use in the United States, despite it being an off-label use for anal fistulas. The high variability regarding the design and methodology of reported studies makes comparison difficult. Few trials were initially prospective and randomized [39, 40], some were prospective and nonrandomized [23, 27, 41–48], while others were retrospective [49, 50]. The patients included in the majority of the trials were usually not standardized. They included patients who had acute and chronic fistulae, Crohn’s disease, HIV-positive patients, postoperative patients, rectovaginal fistulae, and anastomotic fistulae. The commercial preparations of sealant are varied, and the intraoperative protocols differ in terms of preoperative preparation of the patient, management of the fistula in the operating room, and postoperative monitoring. The follow-up was relatively short in many of the trials, although several trials have reported long-term data as can be seen in Table 11.1. As previously described, it is critical to obliterate the entirety of the fistula and any attached branches. For this reason, some authors chose to exclude patients in whom additional tracts were identified [27, 39, 43, 46] or deferred the injection until adequate drainage was achieved [25, 40, 41, 48, 49, 51]. Other investigators chose to include these patients and make attempts to fill all tracts and cavities with sealant [23, 42, 44]. Preoperative antibiotic use was also highly variable in these studies. Authors administered parenteral antibiotics [23, 43, 47], enteral antibiotics [44], or refrained from antibiotic use [39, 41]. There is evidence to suggest that antibiotics mixed within the fibrin sealant will be slowly released from the matrix over 24–48 h [52]. Several studies attempted to improve healing rates based on this laboratory data by including antibiotics within the sealant itself [40, 46]. Table 11.1 contains a summary of available data. Because of the variability in design, a formal systematic review or meta-analysis, although attempted, has not really provided useful information. Nonetheless, a review of the literature is warranted. The world literature review that follows primarily involves studies in which ten or greater patients had some form of fibrin glue treatment. Additionally, on occasion statistics may differ slightly as I thought it would be appropriate to not always dismiss patients who were lost to follow-up but include them on an intention to treat fashion.
Table 11.1
Summary of data over the last 2 decades
Authors | Year | N | Etiology | Success (%) | Type glue | Follow-up | Remarks |
|---|---|---|---|---|---|---|---|
Hjortrup et al. [27] | 1991 | 23 | Crypto, postoperative | 74 | Commercial | 12–26 m | First series including fistula-in-ano in 8 pts, nonrandomized |
Abel et al. [23] | 1993 | 10 | Crypto, RVF, HIV, Crohn’s | 60 | AFTA-C | 3–12 m | Safe and effective, nonrandomized |
Venkatash et al. [47] | 1999 | 30 | Crypto, RVF, HIV, Crohn’s, urethro-vesicorectal | 60 | AFTA-C | 9–57 m | Only recurrent pts enrolled, prospective |
Aitola et al. [42] | 1999 | 10 | Crypto | 0 | Commercial | 6 m | Pilot study |
Cintron et al. [25] | 1999 | 26 | Crypto, Crohn’s | 85 | AFTA-E | 3.5 m | Third-generation autologous |
Nelson et al. [7] | 2000 | 10 | Crypto | 50 | Commercial and dermal advancement flap | 28 (4–63) | Dermal advancement flap and glue odds ratio for recurrence 4.3 |
Cintron et al. [41] | 2000 | 26—A | Crypto, HIV, RVF, Crohn’s | 54 | Autologous or commercial | 12 | Less efficacy in complex fistulae, failure seen 11 m |
53—C | 64 | ||||||
Patrlj et al. [46] | 2000 | 69 | Crypto | 74 | Commercial and cefotaxime | 18–36 | More effective in tracts ≥3.5 cm |
El-Shobaky et al. [45] | 2000 | 30 | Crypto | 87 | Autologous | ? | |
Sentovich [53] | 2001 | 20 | Crypto, Crohn’s | 85 | Autologous/commercial | 10 | |
Lindsey et al. [39] | 2002 | 19 | Crypto, Crohn’s | 63 | Commercial | 3 | Sealant better for complex fistulae Randomized, controlled |
Chan et al. [44] | 2002 | 10 | Crypto | 60 | Commercial | 6 | Prospective nonrandomized |
Tinay et al. [54] | 2003 | 19 | Crypto | 78 | Commercial | 12 | Prospective, nonrandomized |
Sentovich et al. [48] | 2003 | 48 | Crypto, Crohn’s, | 69 | Commercial | 22 (6–46) | Better healing in shorter tracts, 89 % success if retreated, bowel preparation |
Zmora et al. [49] | 2003 | 24 (1°) | Crypto, Crohn’s, postoperative | 33-alone | Commercial | 12.1 (1–36) | Retrospective, Sealant and flap yielded 54 % healing |
13 (flap and glue) | 54-flaps | ||||||
Buchanan et al. [43] | 2003 | 22 | Crypto | 14 | Commercial | 14 | Prospective |
Loungnarath et al. [50] | 2004 | 42 | Crypto, Crohn’s postoperative | 31 | Commercial | 26 | Retrospective 3 pts lost to f/u |
Jurczak et al. [55] | 2004
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





