A basic understanding of female urethral anatomy is necessary to approach urethral reconstruction from an anatomic standpoint. This article reviews the techniques of female urethral reconstruction based on these anatomic divisions: proximal and bladder neck, midurethra, and distal urethra.
The female urethra is relatively short compared with its male counterpart and is generally between 2 and 4 cm in length. It is made up of an inner layer of mucosal epithelium with numerous infoldings that create an effective seal against the passive loss of urine. Beneath the mucosa lies a rich, vascular network of elastic tissue that is much like the corpus spongiosum. Surrounding the spongy vascular tube is a collagen-rich fibromuscular envelope comprising the periurethral fascia. These three components of a normal urethra are crucial in maintaining continence and enabling dynamic function during increases in abdominal pressure and during normal micturition.
Two fascial attachments suport the urethra: the pubourethral and urethropelvic ligaments. The pubourethral ligaments are a band of fascial support located superiorly between the urethra and pubic symphysis. These ligaments often serve as a point of anatomic division between the proximal and distal urethra. The urethropelvic ligaments are comprised of two layers of fascial condensation, the endopelvic fascia and the pubocervical fascia, which provide lateral attachment to the arcus tendineus. The midurethra is believed to be the center of continence where the striated sphincter complex maintains active and passive tone.
A basic understanding of female urethral anatomy is necessary to approach urethral reconstruction from an anatomic standpoint. This article reviews the techniques of female urethral reconstruction based on these anatomic divisions: proximal and bladder neck, midurethra, and distal urethra.
Bladder neck and proximal urethra
The bladder neck and proximal urethra are made up primarily of smooth muscle that is oriented in a circular fashion. This intrinsic, involuntary sphincter maintains passive continence in addition to the striated urethral sphincter. Failure of the bladder neck sphincteric mechanism can be related to age, childbirth, neurologic disease, congenital anomalies, and pelvic surgery. Intrinsic sphincter deficiency is a common finding in women with stress incontinence and is apparent as funneling of the bladder neck during the filling phase on cystography. Reconstruction of the bladder neck to restore continence can be accomplished in several ways depending on the severity of incontinence, its underlying cause and associated anatomic defects, and the goals of surgical correction. This article describes the technical aspects of bladder neck reconstruction in women, specifically pubovaginal sling placement, surgical closure of the bladder neck, and placement of an artificial urinary sphincter.
Pubovaginal Sling
Traditionally, pubovaginal sling surgery has been used to treat intrinsic sphincter deficiency and stress urinary incontinence, with concomitant correction of hypermobility and restoration of posterior urethral support. Sling materials include autologous fascia, allografts, xenografts, and synthetics. The most common pubovaginal sling technique involves harvesting a strip of autologous fascia, generally rectus abdominis fascia or fascia lata, which is fashioned as a 2 cm × 10- to 15-cm strip. This technique is considered the gold-standard surgical treatment of genuine stress urinary incontinence.
With the patient in the dorsolithotomy position, an incision is made in the anterior vaginal wall at the level of the bladder neck, identifiable by palpation of a Foley catheter balloon. A submucosal tunnel is created beneath the bladder neck and is extended to the endopelvic fascia laterally, which subsequently is perforated to accommodate passage of the sling into the retropubic space. The autologous fascial sling is prepared by passage of nonabsorbable suture through either end. After closure of the fascial harvest site, the sling is placed beneath the bladder neck and passed into the retropubic space above the rectus abdominis fascia. Passage of the sling sutures into the retropubic space can be accomplished using a double-pronged passer (such as the Raz-Peyrera) or a long clamp under finger guidance, taking care to avoid medial deviation. The bladder should be relatively nondistended to avoid inadvertent cystotomy. A cystoscopy should be performed after sling suture passage to ensure bladder integrity and to assess for the presence of any suture material within the bladder lumen. The sling sutures are tied loosely in the midline, above the rectus abdominis fascia, to avoid excessive tension on the bladder neck. A Foley catheter is left in place after surgery until normal voiding ensues, generally between several days to several weeks.
Modifications to this technique include the use of bone anchors for sling fixation, various materials for sling preparation (ie, allograft, xenograft, synthetics), and in situ suspension of the vaginal wall sling. Numerous modifications to the traditional autologous sling technique have been devised to reduce the morbidity associated with autologous fascial harvest. The specifics of these modifications are discussed elsewhere in this issue.
Closure of the Transvaginal Bladder Neck
Surgical closure of the bladder neck in females is indicated in cases of extensive urethral destruction, usually because of a chronic indwelling urethral catheter in cases of neurogenic bladder. Progressive urethral dilatation leads to chronic, intractable incontinence around an indwelling catheter, with associated perineal excoriation and chronic urinary tract infection. Bladder neck closure can be readily accomplished using a transvaginal approach, thereby obviating the need for a complete urinary diversion or transabdominal closure. The bladder then can be managed with an indwelling suprapubic catheter unless a catheterizable stoma is desirable. This technique is analogous to perineal closure of the male urethra for refractory incontinence.
Transvaginal bladder neck closure is accomplished in the dorsolithotomy position. Initially, the surgeon can place a suprapubic cystotomy unless another form of bladder drainage has been established, such as a continent stoma (ie, Mitrofanoff) or an incontinent stoma (ie, ileal bladder chimney). A simple, percutaneous suprapubic cystotomy can be performed using a curved Lowsley retractor or by open technique if indicated. Slight traction on the suprapubic catheter prevents bleeding from the cystotomy and extravasation of urine into the retropubic space.
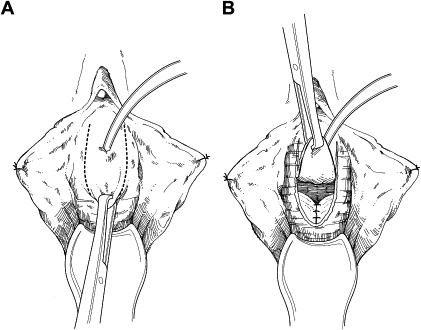
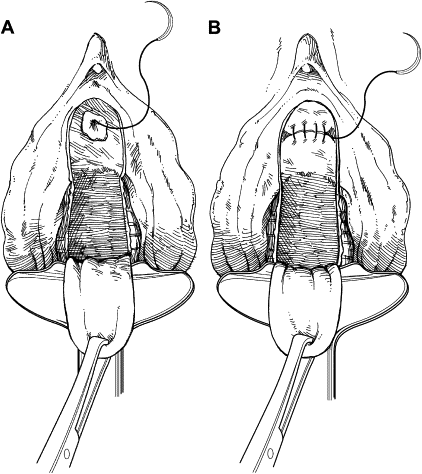
The anterior vaginal wall is incised, circumscribing the damaged urethra and extending onto the anterior vaginal wall in an inverted U-shaped configuration ( Fig. 1 ). This approach allows the creation of an anterior vaginal wall flap by dissection from the underlying perivesical and periurethral fascia. Dissection of the bladder neck is extended laterally to the endopelvic fascia and pubic rami. The endopelvic fascia is perforated sharply to allow adequate mobilization of the bladder neck and base ( Fig. 2 ). Indigo carmine is administered intravenously to allow cystoscopic visualization of the ureteral orifices and their proximity to the bladder neck.
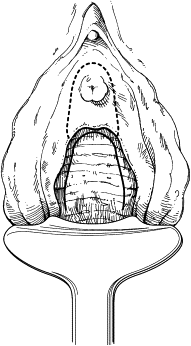
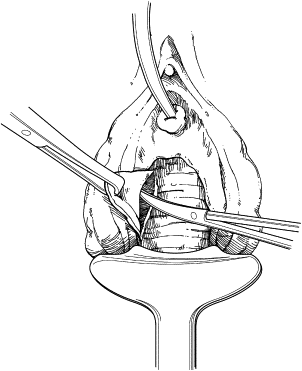
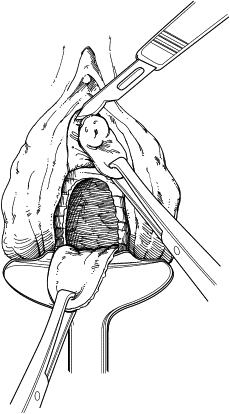
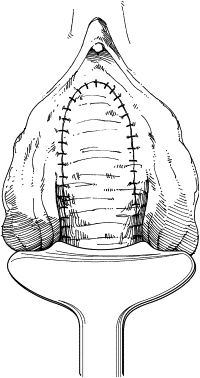
The remaining damaged urethra is excised completely, and closure of the bladder neck is performed ( Fig. 3 ). A running suture of absorbable material is placed in the mucosa, followed by a second layer of suture placed in the perivesical fascia and muscular bladder wall ( Fig. 4 ). The second suture line extends from the bladder neck to the anterior bladder wall behind the pubic symphysis, thereby placing the closed bladder neck in the retropubic space. This step avoids direct apposition of suture lines, which may lead to fistula development. The anterior vaginal wall flap is advanced as a third layer ( Fig. 5 ). If the vaginal and perivesical tissues are compromised secondary to chronic infection or before radiation therapy, additional tissue interposition can be accomplished by use of a Martius labial fat pad flap. A vaginal pack impregnated with antibiotic cream is left for 24 hours after surgery. The native bladder then can be managed with a chronic, indwelling suprapubic cystotomy or creation of a catheterizable stoma with augmentation cystoplasty in cases of reduced capacity, impaired compliance, or vesicoureteral reflux.
In certain circumstances, it may be desirable to perform bladder neck closure by an abdominal approach. This step can be done transvesically or extravesically.
Artificial Urinary Sphincter
Although commonly used in males with stress urinary incontinence, the artificial urinary sphincter (AUS) has had limited use in females and generally is reserved for treating extreme cases of incontinence involving multiple failed anti-incontinence operations (type III stress incontinence), neuropathic bladder dysfunction, or congenital anomalies. There have been reports of experience with and outcomes of AUS in female children and adolescents with underlying neuropathic bladder or exstrophy–epispadias complex. Long-term continence after AUS placement has been reportedly lower in females than in males, and females have an associated increased risk for AUS explantation. Previous pelvic radiation therapy is a contraindication to AUS placement in females because of the high risks for erosion and device explantation.
AUS placement in females can be performed transabdominally or transvaginally with placement of the cuff at the bladder neck. Broad-spectrum intravenous antibiotics that are targeted at coverage of gram-positive skin flora, gram-negative organisms, and anaerobes are administered on the day of surgery and after surgery. Urine must be sterile before surgery. The avoidance of a urethral catheter before surgery is ideal to minimize infection. Meticulous surgical technique is also necessary to reduce postoperative morbidity.
Transabdominal approach
In women, the retropubic, extraperitoneal approach to AUS placement most commonly is employed. The retropubic space generally is entered through a lower abdominal or Pfannenstiel incision, and mobilization of the bladder neck, distal to the ureteral orifices, is performed. Posteriorly, the vesicovaginal plane carefully is dissected just superior to the endopelvic fascia. Any incidental cystotomy or vaginal opening should be sutured in two layers with absorbable suture material to achieve a watertight closure.
The cuff sizer is applied to measure the circumference of the bladder neck, allowing selection of a cuff that is not excessively tight or loose. The cuff is passed carefully around the bladder neck and locked, avoiding the use of sharp instruments in this area. The AUS pump generally is placed within the labia majora, and the balloon reservoir is placed in the preperitoneal rectus abdominis muscle space. The system is cycled after all connections have been made and is left deactivated. A Foley catheter is left in place overnight.
Transvaginal approach
The transvaginal approach to AUS placement in women allows dissection of the urethrovaginal plane under direct visualization. This plane can be obliterated or extensively scarred after previous anti-incontinence procedures, making the transabdominal approach to dissection challenging and resulting in an associated risk for urethral, bladder neck, or vaginal injury.
A vertical incision is made in the anterior vaginal wall from the level of the midurethra to the proximal bladder neck. The vaginal flaps are dissected free laterally toward the endopelvic fascia and should be thick, as they will provide coverage of the AUS. The urethra and bladder neck are freed from their lateral attachments to the pelvic floor musculature and vaginal wall and from their anterior attachments to the pubic symphysis. In cases of extensive scarring, an additional suprameatal incision can be made to allow better access to the anterior surface of the urethra and bladder neck. Ultimately, a circumferential dissection of the bladder neck is accomplished in preparation for cuff placement.
The device is left deactivated at the time of operation, and patients return between 4 and 6 weeks after surgery for AUS activation. Thomas and colleagues reported their experience with 68 female patients undergoing AUS placement. They used a 71- to 80-cm water balloon, except in cases of significant bladder neck scarring, atrophy of tissues, or decreased vascularity, in which they used a 61- to 70-cm water balloon. Some investigators advocate the use of a lower-pressure reservoir (51–60 cm of water) in women to prevent device erosion. In general, patients with genuine stress incontinence (type III) alone have a better long-term prognosis with AUS placement and have significantly decreased rates of explantation, whereas patients with neuropathic incontinence exhibit higher rates of infection and device erosion (approximately 50%), requiring explantation. The rates of continence after AUS placement in the neuropathic population remain high (approximately 90%).
Midurethra
The midsegment or proximal segment of the female urethra, between the true bladder neck and pubourethral ligaments, contains the striated sphincter complex and levator ani insertion. The authors believe that the most critical components of active and passive continence are located in this important segment of urethra. Sling surgery for stress incontinence has been targeted at the midurethra rather than the bladder neck, where pubovaginal slings have been placed traditionally. Placement of sling material in the midurethra causes scarring and fixation of the urethra to the pubic bone, thereby recreating the damaged or attenuated pubourethral ligaments. The sling material beneath the urethra reinforces the suburethral vaginal hammock, protecting against incontinence during increases in abdominal pressure. Intrinsic sphincter deficiency and urethral hypermobility can be treated with a midurethral sling, and this approach has excellent long-term outcomes that are comparable with outcomes for the traditional pubovaginal sling.
Intrinsic damage of the midurethra can lead to stress incontinence, total incontinence, or stricture with resultant obstruction. The treatment of stress and total incontinence is discussed elsewhere in this issue. This discussion focuses on reconstruction of the female midurethra for cases of stricture, sling erosion, and urethrovaginal fistula. Stricture of the female urethra is caused by radiation therapy for pelvic malignancies, previous urethral instrumentation or endoscopic surgery, trauma, iatrogenic injury during urethral diverticulectomy, and gynecologic surgery. Increasing numbers of anti-incontinence surgeries and the widespread use of synthetic sling materials have made urethral erosion a well-recognized morbidity, requiring sling removal and urethral reconstruction. Urethrovaginal fistula related to prolonged childbirth is a common finding in underdeveloped countries, but this complication also can occur after urologic and gynecologic surgery.
Urethral Reconstruction for Stricture
The treatment algorithm for female urethral stricture is not as well defined as the male counterpart. This discrepancy may be attributable to the relative rarity of stricture disease in women, especially in cases of blunt pelvic trauma. Because of the female urethra’s short length, anatomic position behind the pubic arch, and relative mobility, the incidence of stricture that occurs after trauma in females is low (range, 0%–6%). More commonly, stricture disease in women is seen after pelvic radiation therapy for gynecologic malignancies and can occur many years later. Generally, repair of stricture disease is divided into endoscopic and open repairs, with the use of local tissue flaps or graft interposition. Because of the relatively short length of the female urethra (approximately 4 cm), stricture excision and end-to-end urethroplasty are not feasible as in male patients.
Endoscopic repair
Midurethral stricture can be treated endoscopically with a cold knife or a Holmium laser. The laser technique requires use of a large-diameter (500–1000 μm) fiber and high-energy and high-frequency settings for effective tissue penetration and cutting. Incisions are performed at the 3 o’clock and 9 o’clock positions with an additional 12 o’clock incision when necessary. A urethral catheter, generally sized 16 Fr or larger, is left in place for several days after surgery. Use of clean intermittent catheterization after surgery can help reduce the risk for recurrence; however, the ideal regimen and duration have not been well defined.
In severe cases of midurethral stricture that are not amenable to simple optical urethrotomy as previously described, a “cut to the light” procedure can be performed. This procedure necessitates placement of a flexible cystoscope in an antegrade fashion to help in the identification of the obliterated urethral segment and the true bladder neck. Such complex cases require prolonged postoperative catheterization to ensure complete healing and re-epithelialization.
Open repair: flap urethroplasty
Vaginal flap urethroplasty can be used to recreate a functional urethra by way of local, healthy tissues. This technique also can improve urethral length in cases of a shortened urethra that is associated with vaginal voiding. A flap of vaginal epithelium in a U-shaped configuration can be employed as a patch or posterior plate of tissue ( Fig. 6 A). A vertical incision in the anterior vaginal wall located directly beneath the urethra allows adequate dissection of the urethra. A longitudinal incision in the posterior urethra is made, effectively exposing the entire segment of strictured or diseased urethra, until more proximal and viable tissue is identified. The vaginal flap is harvested by incising the more cephalad, anterior vaginal wall in a U-shaped configuration, approximately 1.0 to 1.5 cm in width. This flap is flipped up and sutured to the edges of the open posterior urethra using absorbable suture material ( Fig. 6 B). The anterior vaginal wall is closed primarily, creating a second layer of tissue beneath the newly created urethra. In cases of compromised anterior vaginal wall tissue, coverage can be attained by use of labial or local thigh flaps. An additional technique of vaginal flap urethroplasty has been described in which bilateral incisions that are parallel and adjacent to the urethral meatus are used. Vaginal wall flaps are dissected laterally on either side of the urethra and are sutured together in the midline over a 14-Fr urethral catheter ( Figs. 7 A and B).