Fig. 18.1
Normal fallopian tube. Salpingoscopy reveals normal-looking mucosa in the ampullary (a) and infundibular (b) regions. A thin section of the ampulla (c) shows normal ciliated endosalpinx. Electron microscopy (d) shows evidence of normal homogenous cytoplasm, abundant mitochondria, columnar-shaped cells, normal nuclei, and a normal complement of cilia. (Reprinted from Hershlag et al. ,1991 with permission from Wolters Kluwer Health)
Ciliated epithelium has adhesive features that assist in ovum transport. Cilia beat in the direction of the uterus. This is the site of ovum capture and accounts for distal tubal dysfunction. The ampulla is 5–8 cm long and is the site of fertilization and early cleavage of the human embryo. This is also the most common site of tubal ectopic pregnancies.
The isthmus extends from the ampulla to the uterus and is the site of proximal tubal obstructions, which may inhibit spermatozoa from reaching the egg. The length of the isthmus is 2–3 cm and is the most muscular portion of the fallopian tube with the narrowest lumen. The mucosa is arranged in folds and is less ciliated compared with the distal tube. The intramural segment is the portion of tube that extends into the uterine cavity. It has three layers of muscle and has been described as the sphincter between the uterus and tube.
Tubal Fluid
Tubal fluid plays a critical role in providing the right environment for gametes traveling through the tube and optimizing circumstances for sperm capacitation and fertilization of the egg. In addition, in the past decade, evidence from in vitro fertilization (IVF) data has shed light over the role of tubal fluid in implantation. Specifically, IVF in patients with hydrosalpinx results in a ~ 50 % reduction in pregnancy rates compared with patients without tubal disease [4]. In patients in whom the diseased tubes have been surgically removed, pregnancy rates have improved back to control values [5, 6]. Follicular fluid (FF) has been shown to be toxic to embryos in vitro [7]. Tubal fluid is composed of electrolytes, specifically high levels of potassium and bicarbonate compared with plasma. In addition, glycoproteins, glucose, pyruvate, lactate, and 17 different amino acids have been identified in tubal secretions [8]. The concentration of glucose fluctuates with the menstrual cycle. Following ovulation , the concentration of glucose decreases tenfold [9]. Tubal lumen glucose concentration drops from 3.1 mM in the follicular phase to 0.5 mM midcycle [10]. Human and animal studies have shown that estrogen and progesterone modulate oviductal fluid secretion by the epithelium [11, 12]. Estrogen causes hypertrophy, maturation, and the differentiation of the ciliated phenotype in fallopian tube epithelial cells in vitro [13]. Conversely, progesterone causes atrophy and decreases secretory production during the luteal phase .
Tubal fluid also contains glycoproteins, which may have a role in early development of the oocyte and embryos, enhance adherence of spermatozoa to the isthmus of the fallopian tube, and improve fertilization . Prior to ovulation, an oviduct-specific glycoprotein (OSGP) has been identified to enhance sperm capacitation, bind to the zona pellucida, and facilitate sperm penetration [14]. Others have postulated that OSGP and other glycoproteins increase the viscosity of the oviductal fluid, which serves as a buffer for the embryo against osmotic changes in the luminal fluid and also as protection against immunologic factors [15].
Spermatozoa undergo capacitation and antigenic changes through exposure to the uterus and tubal fluid, containing copious amounts of β-amylase [16]. This step is followed by the acrosome reaction, hyperactivation, and successful penetration of the zona pellucida of the oocyte.
In conclusion, the delicate composition of tubal fluid plays a critical role in fertilization of the egg, as well as embryo development and transport to the uterus. Any disturbance in the production of tubal fluid due to an erratic hormone milieu, destruction of secretory cells by fibrosis, or infection could potentially lead to altered sperm and egg transport and fertilization .
Tubal Inflammation
It is unclear what proportion of acute episodes of salpingitis is subclinical. Many young women may experience no symptoms or a low-grade fever or nonspecific abdominal pain. Oftentimes, these women do not seek medical care or get misdiagnosed. Physicians should therefore have a low threshold for diagnosis and treatment of subclinical or “silent” salpingitis to prevent future ectopic pregnancies and infertility.
Subclinical Infection
In the 9 months following HSG and/or laparoscopy in ovulatory women with patent tubes, patients with Chlamydia trachomatis IgG seropositivity have a 33 % lower conception rate compared with seronegative patients [17]. The changes caused by C. trachomatis are intraluminal, and histological changes have been observed by salpingoscopy and biopsies of luminal epithelium (Figs. 18.2 and 18.3). Normal architecture of the fallopian tube is lost as neutrophils invade the tubal plicae and cause edema and congestion acutely. Long-term sequelae include scarring, fibrosis, and loss of luminal folds, normal epithelium, and cilia [18]. In addition, antibodies from a chronic inflammatory reaction elicits an autoimmune response to release human heat shock proteins (HSPs) [19]. These human HSPs can have a negative influence on the developing embryo during tubal transport as well as during implantation.

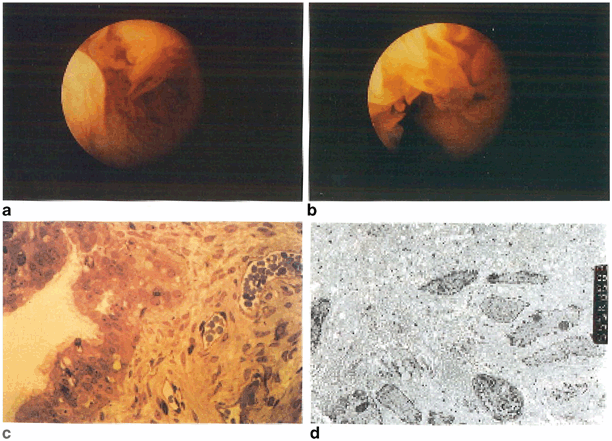

Fig. 18.2
Salpingoscopic view of a normal ampulla (a, b). Black and white arrows denote primary and secondary folds, respectively (b). An intraluminal adhesion is shown in the isthmus (c) via flexible salpingoscope. The ampullary lumen in a patient with severe hydrosalpinx (d) is devoid of primary and secondary folds, with abnormal vessel formation. (Reprinted from Hershlag et al. 1991, with permission from Wolters Kluwer Health)

Fig. 18.3
Moderate tubal disease. Rigid salpingoscopy shows moderate attenuation of the epithelial folds (a) and increased vascularity (b). Histology (c) of the ampulla shows evidence of epithelial proliferation, thickening of the lamina propria, and increased vascularity. Electron microscopy (d) shows broken plasmalemma, swollen mitochondria, and vacuolated cytoplasm. (Reprinted from Hershlag et al. 1991, with permission from Wolters Kluwer Health)
Acute Salpingitis
Acute salpingitis is usually secondary to sexually transmitted infection by Neisseria gonorrhoeae, C. Trachomatis, Mycoplasma, streptococci, staphylococci, coliform bacilli, and anaerobes, which reach the tube usually directly from the uterus attached to sperm or by blood vessels or lymphatic drainage. Clinically, young women may experience fever, leukocytosis, and adnexal tenderness [20]. The tube is usually enlarged, erythematous, and edematous. There can be exudates and an associated tubo-ovarian abscess. Microscopically, there are numerous neutrophils, congestion, and edema.
Chronic Salpingitis
Chronic salpingitis frequently goes unrecognized. In patients with no known antecedent history of sexually transmitted diseases (STDs), 35.9 % of them tested positive for C. trachomatis antibody titer and had tuboperitoneal abnormalities found during an infertility workup [21]. What is better to have: a diagnosed or an undiagnosed salpingitis? This is yet another question that has not been fully studied. While the acute salpingitis may be more severe, because of symptoms, it is usually promptly treated with aggressive antibiotics, thus allowing some tubes to heal without significant scarring, while others may not escape long-term damage. The undiagnosed and therefore untreated kind, on the other hand, may represent a lower level of inflammation, but it is yet to be determined whether it could more likely to cause permanent tubal dysfunction secondary to subtle chronic changes in tubal anatomy as well as contractile motility.
The chronically inflamed tube can become enlarged, distorted, or adherent to other pelvic organs. There can be hydro- or pyosalpinx in which the tube is full of fluid or exudates, respectively. Microscopically, the tubal folds may be shortened, blunted, or fibrotic (Fig. 18.3). There is a chronic inflammatory infiltrate and flattening of the epithelial lining (Fig. 18.4).
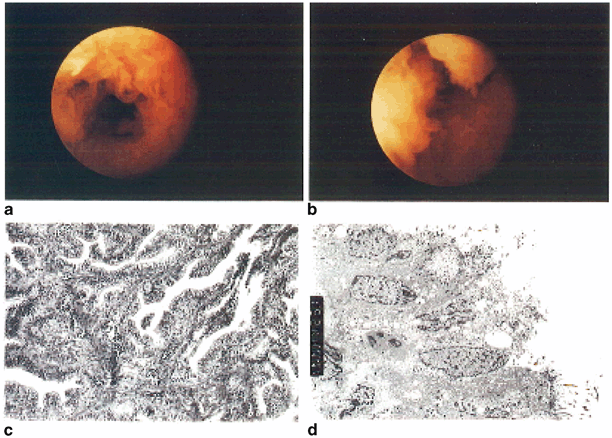

Fig. 18.4
Moderate tubal disease. Rigid salpingoscopy shows attenuation of ampullary epithelial folds (a) with absence of secondary folds and widening of spaces between primary folds (b). Histology (c) of the infundibulum of the same tube shows evidence of conglutination and muscular hypertrophy of the fimbrial stalk. On electron microscopy (d), the epithelial cells are cuboidal in shape, with sparse numbers of cilia per cell. The nuclei are swollen and large vacuoles are present. (Reprinted from Hershlag et al. 1991, with permission from Wolters Kluwer Health)
Isthmic Plugs
Since the isthmus is less than 1 mm in diameter compared with the ampullary portion, which is 1 cm wide, mucus plugs may represent a relatively common and frequently unrecognized underlying cause of unexplained infertility.
Histologically, endotubal isthmic plugs consist of casts of histiocytes mixed with endometrial stroma or mesothelial cells [22]. Several studies have observed isthmic secretions increase with estrogen exposure and decrease after ovulation as the corpora lutea forms. Hypotheses on the origin of these isthmic plugs include retrograde menstrual flow, a sign of early pelvic infection, or secondary to cyclical estrogen prior to ovulation .
There are several lines of evidence that mucus plugs may play a major role in unexplained infertility. In a study by Sulak et al. [23], the majority of fallopian tubes diagnosed as obstructed by HSG were found to have only a mucus plug. Successful tubal cannulation either through hysteroscopy or novy cornual cannulation may also be partially attributable to dislodging a mucus plug rather than dilating an obstructed tube [24]. Flushing of tubal plugs could be the reason why for many years it has been known that performing an HSG demonstrating tubal patency is associated with a relative increase in fecundability of up to 20 % [25]. In many patients, dislodging the mucus plug may be all it takes to reopen the sperm–egg highway. Unfortunately, identifying the presence of a mucous plug prior to dislodging it during an HSG is not possible.
Salpingitis Isthmica Nodosa
Salpingitis isthmica nodosa, or SIN, is an old HSG diagnosis for a nodular proximal tubal occlusion. The fallopian tube has nodular thickening of the tunica muscularis in the proximal isthmus enclosing dilated glands, which causes complete obliteration of the lumen [26]. The incidence of SIN in healthy fertile women ranges from 0.6 to 11 %, with a mean age of 26 years, and predisposes patients to infertility and ectopic pregnancies [27, 28]. It is bilateral in 85 % of cases [29]. The etiology is unknown, but some hypothesize a postinflammatory effect [30]. On salpingoscopy , this entity may present with no apparent lesion or gray–white nodules up to 2 cm in size in the isthmic portion of the fallopian tube. Microscopically, one can see nodules, cystic and dilated tubal epithelium with hypertrophic muscularis, and normal tubes distally. SIN is considered a risk factor for ectopic pregnancy [31].
How relevant is the diagnosis of SIN in the IVF era? The incidence of SIN in women with an ectopic pregnancy is 10 % compared with 0.2 % in the control group [32]. Patients with bilateral SIN should not attempt repair since the success rate is extremely poor with a high risk of ectopic pregnancy, and these patients should be immediately referred to IVF.
Granulomatous Salpingitis
Tuberculosis was probably at one point the most common reason of irreversible infertility in endemic areas, giving the typical lead pipe appearance on HSG. Genital tuberculosis involves the fallopian tubes bilaterally in 90 % of women [33]. The most common cause of granulomatous salpingitis is Mycobacterium tuberculosis or Mycobacterium bovis. Grossly, there is thickening and nodularity in the wall, serosal tubercles, or caseous luminal exudate. In severe forms, there may be adhesions between the ovaries and other organs in the pelvis. Interestingly, the ostium usually remains open in contrast to many other forms of chronic salpingitis. Histologically, the mucosa has caseating granulomas, inflammatory changes, and fibrosis. There are epithelial hyperplasia and Schaumann bodies. Other less common causes of granulomatous salpingitis include leprosy [34], Crohn’s disease [35], sarcoidosis [36], chronic pelvic inflammatory disease [37], endometriosis [38], pelvic radiation [39], and giant cell arteritis [40].
Patients who had an intrauterine device (IUD) are most at risk for actinomycosis of the fallopian tubes, a disease caused by the Actinomyces species such as Actinomyces israelii. It affects both tubes in 50 % of cases, which may spread to the ovaries [41]. It is estimated that 7 % of women using an IUD have colonization with an Actinomyces species on a cervical smear [42]. If a patient is asymptomatic, there is no need for antimicrobial treatment or IUD removal. However, if a woman with an IUD has pelvic symptoms and a positive cervical smear for A. israelii, they are four times more likely to develop pelvic inflammatory disease [43]. Diagnosis can be made with Pap smear, computed tomography-guided tissue biopsy [44], or laparoscopically with a culture from the fimbrial lumen or posterior cul-de-sac [45]. On gross inspection, there can be small yellow flecks composed of sulfur granules within the tubal lumen. Sequelae of this infection include fistula communication between bowel, bladder, or skin. Histological examination shows granules of gram-positive filamentous bacteria surrounded by purulent exudates [46].
Parasitic Salpingitis
Parasites such as Schistosoma haemotobium have been found to cause nodularity and scarring with the histological finding of characteristic ova surrounded by fibrosis. Pinworms caused by Enterobius vermicularis have been identified as nodular thickening in the tubal wall. They spread from anal infections to the genital tract. The characteristics include eosinophilic infiltrates, giant cells, granulation tissue, and fibrous tissue. Cysticercosis has also been reported in the tube, with its characteristic calcified larvae surrounded by granulomatous salpingitis [49].
Physiological Salpingitis
Physiological salpingitis is a nonbacterial inflammatory reaction that can be found in women at the time of menstruation or the peripartum period [50]. Clinically, patients are usually asymptomatic. The diagnosis was made from routine analysis of tubes removed at the time of hysterectomy or sterilization. Histologically, there is edema, lymphatic dilation, and infiltration with polymorphonuclear lymphocytes in the tubal plicae [51]. However, unlike infectious salpingitis, there is no necrosis, ulceration, or bacterial infection. Menstrual debris causes an inflammatory reaction in the tubal epithelium and stroma, but rarely involve the muscular walls. There are no known long-term sequelae from physiologic salpingitis. However, women are more susceptible to pelvic infections during menses due to low estrogen, less cervical mucous to block ascension of bacteria, and the presence of this physiologic salpingitis could facilitate infectious spread [52].
Endometriosis
Similar to endometriotic lesions of other pelvic organs, it is unclear how the involvement of the fallopian tube in endometriosis affects fertility in most cases . Only a full obstruction of both tubes or unilateral obstruction where the other tube is absent represents a clear association. However, such instances are uncommon. The most common site of endometrial foci in the fallopian tube is the serosa, but the myosalpinx and mucosa can also be involved [53]. Endometrial tissue may spread from the uterus to the isthmic portion of the tube along with mucosal changes such as obstructing endometrial polyps [54]. These intratubal polyps are associated with ectopic pregnancy and infertility, especially if they are bilateral [55]. In one review, the prevalence of intramural tubal polyps was 3.8 % in a group of infertile women, where 50 % had unexplained infertility [56]. The diagnosis can be made by HSG. The lesions are typically broad based, 0.1–1.3cm, pink to red, smooth protrusions from the mucosa. Microscopically, one can see non-functioning endometrium covering the polyp.
In addition to obstructing lesions, endometriosis causes elevated oxidative stress, increased immunologic factors, and changes in the hormonal levels in peritoneal and FF. Reactive oxidative species (ROS) produced by erythrocytes, macrophages, and apoptotic endometriotic cells induce oocyte degeneration, DNA damage , increased cell membrane permeability [57], and cell death [58]. ROS negatively impacts fertilization through increased DNA fragmentation in spermatozoa [59] and inhibition of the acrosome reaction [60]. Nitrous oxide (NO) is higher in patients with endometriosis from the presence of macrophages. NO is toxic to embryos and decreases sperm motility [61].
Endometriosis has also been shown to affect the sperm interaction with the tubal epithelium. The tubal ampulla binds significantly more spermatozoa in women with endometriosis leaving less freely motile spermatozoa for fertilization [62]. Another study found cilia to beat at a lower frequency when exposed to peritoneal fluid from women with endometriosis [63].
Endometriosis may impair fertilization and implantation through decreasing oocyte quality, decreasing sperm motility, and/or exposure of the gametes/embryo to toxic peritoneal and FF. The FF of women with endometriosis decreases sperm binding to the zona pellucida [64]. Women with endometriosis have decreased levels of vascular endothelial growth factor (VEGF), which has been associated with reduced embryo quality and implantation defects [65]. Increased levels of circulating immunoglobulins and complement deposits have been detected in the peritoneal fluid of patients with endometriosis. This local inflammatory cascade has been proposed to increase ectopic endometrial implants, promote its growth, and release free radicals .
There are increased E2 levels in peritoneal fluid that stimulates cycle-oxygenase-2 (COX-2) enzyme, which then upregulates prostaglandin E2 (PGE2) production. PGE2 stimulates aromatase expression in endometrial tissue, which then produces more E2 and continues a vicious cycle of proliferation and cytokine induction [66]. Specifically, interleukin (IL)-6, and IL-1a cytokine produced by endometrial and epithelial cells in response to E2, has been shown to decrease sperm motility within the uterus [67].
The impact of endometriosis on fertility is widespread and still has many unanswered questions. Even with fertility treatments such as IVF, pregnancy rates are lower in women with endometriosis compared with controls (tubal factor) [68]. Should these patients be treated like unexplained infertility, especially if the diagnosis was found incidentally? There is still no clear answer for targeted treatment. Differentiating mild from severe endometriosis may help stratify who should be offered laparoscopic treatment, expectant management , or immediate assisted reproduction techniques (ART). The current recommendations are to treat patients with mild endometriosis similarly to patients with unexplained infertility, especially when invasive surgeries have not been shown to have a better clinical outcome [69].
Late Sequelae of Chronic Salpingitis
Fimbrial Agglutination
Fimbrial agglutination can cause distal tubal obstruction and can range from mild adhesions to phimosis, or narrowing of the tube to severe occlusion (Fig. 18.3). Complete occlusion can prevent ovum capture, while phimosis may decrease ovum pick-up. Microsurgery such as fimbrioplasty and neosalpingostomy can open the tubes to restore fimbria and patency.
Intraluminal Fibrosis
Intraluminal fibrosis is a late sequelae of chronic salpingitis and is irreversible. The fallopian tubes appear anatomically patent and normal. The definitive diagnosis of intraluminal fibrosis can only be made with salpingoscopy ; however, most people rely on HSG or laparoscopic tubal lavage to diagnose tubal obstruction, techniques that are incapable of making such a specific diagnosis . A definitive diagnosis can be made by pathologists on analysis of surgically -removed fallopian tubes . According to a study of histological features of surgically removed tubes, 35.5 % of specimens showed plical fibrosis [70]. Mild to moderate fibrosis was found in patients at an average age of 28 years and severe fibrosis at an average age of 42 years. Several studies have shown that there is a steady increase in plical fibrosis and the relative amount of plicae occupying the lumen in the fallopian tube from birth to menopause [71]. These age-groups represent the majority of patients who present for infertility evaluation.
Ciliary Dysfunction
The tubal cilia provide a delicate mechanism of propagating gametes toward the uterus in a unidirectional beat. In a study in rabbits, a segment of the ampulla was reversed so that the cilia beat toward the ovary. The experiment did not affect fertilization , but it did arrest the egg from moving to the uterus, and no pregnancy occurred [72]. Kartagener’s syndrome serves as an interesting model of what happens when the ultramicroscopic structure of the cilia is abnormal (congenital absence of dynein arms) [73]. This rare syndrome may, at times, be associated with female subfertility , ectopic pregnancy, and male infertility with impaired sperm motility [74]. While patients with Kartagener’s syndrome are natural candidates for ciliary dysfunction and therefore male and female infertility , they do not have an increased rate of ectopic pregnancies documented in the literature. Pregnancies in women with this syndrome have been reported, which highlights the importance of the tubal musculature and peristalsis to facilitate oocyte transport to the uterus.
Ciliary function changes during the menstrual cycle, depending on the relative concentrations of estrogen and progesterone, with variations in the respective receptors. In vivo studies show that progesterone decreases ciliary beat frequency (CBF) by 40–50 %, which correlates to the proliferative phase allowing maximal fertilization time of the ovum. Estradiol inhibits the antagonistic effect of progesterone on CBF. From these studies, it can be hypothesized that ovulatory dysfunction could lead to defective CBF and therefore inhibit transport of the ovum along the fallopian tube . At this point, the question of whether ovulation induction agents such as clomiphene citrate and/or gonadotropins could fail because of their adverse affect on ciliary function remains unanswered.
Tubal Motility
It is unclear whether cilia or tubal muscular contractions provide the propulsive force to transport sperm and the oocyte as the data are conflicting. In one study, interference with muscular contractility in the rabbit did not block egg transport [75]. In another study on rabbits, blocking smooth muscle with nicardipine caused oviduct stasis and inhibited egg movement even with unaffected ciliary beating [76]. In addition, reversal of a segment of the ampulla so that cilia beat in the other direction did not block fertilization but did arrest egg transport [77]. Therefore, both the myosalpinx and cilia beating are critical for egg transport. At ovulation , waves of intermittent smooth muscle contraction in the myosalpinx move the egg from the infundibular ostium to the ampullary-isthmic junction, in a prouterine direction [78]. Cilia in the isthmus beat toward the ampullary-isthmic junction at the time of ovulation [79].
After fertilization, the musculature of the distal isthmus relaxes and the egg passes into the uterus. The isthmic musculature (adrenergic innervation) is upregulated by estrogen prior to ovulation, allowing for constriction and therefore retention of the egg for adequate fertilization time in the ampulla [80]. Progesterone binds to β-adrenergic receptors and causes isthmic smooth muscle relaxation [81]. In theory, cases of tubal spasm can be attributed to dysregulation of the myosalpinx by erratic steroid hormone production in women with irregular ovulatory cycles. In a study of human fallopian tubes , muscular contractions were significantly increased by PGE2 and F2α and downregulated by progesterone, levonorgestrel, mifepristone, oxytocin, and human chorionic gonadotropin [82].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





