Fig. 40.1
Eight potential spaces of the pelvis: retropubic space of Retzius, paravesicle spaces (2), vesicovaginal space, pararectal spaces (2), rectovaginal space, and presacral space (Reprinted with permission from: Bristow et al. [55])
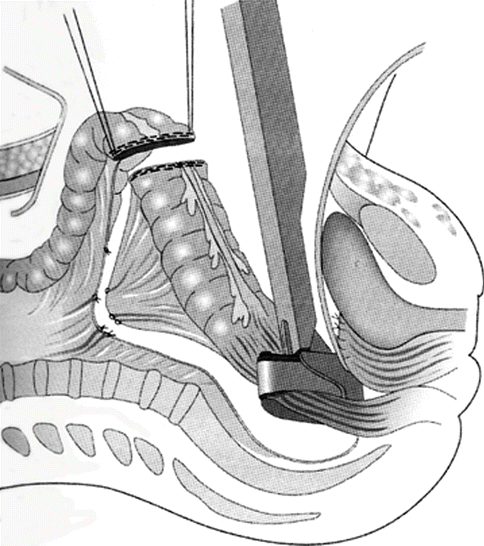
Fig. 40.2
Radical oophorectomy: type II modification. The rectum is divided between a TA stapler and proximal bowel clamp to complete the resection. (Reprinted with permission from: Bristow et al. [55])
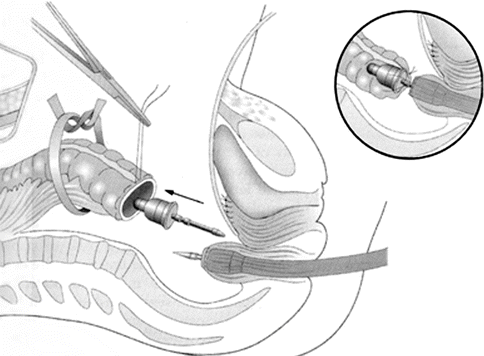
Fig. 40.3
Circular end-to-end stapled anastomosis using the automated CEEA stapler. The CEEA anvil is introduced into the proximal colon and the purse-string suture tied with the notch on the anvil shaft; the main CEEA instrument is passed transanally and the trochar advanced through the rectal stump. The trochar is removed and the anvil shaft inserted into the cartridge shaft of the main CEEA instrument (inset). Abbreviation: CEEA, circular end-to-end anastomosis. (Reprinted with permission from: Bristow et al. [55])
Series of patients undergoing en bloc posterior pelvic resection have been reported from multiple centers with acceptable complication rates, and are listed in Table 40.1 [16, 18–26]. In patients undergoing several separate cytoreductive procedures, the “per procedure” complication rate can be difficult to determine. In these series, the procedure-related complication rate was generally 2–6 %, the most common of which was anastomotic leak or fistula. Houvenaeghel and colleagues reported the combined experience of nine French centers, in which 168 patients underwent modified posterior exenteration in the primary setting with a low rate of protective stoma [25]. The total perioperative complication rate was 27 %, with a low rate of fistula/abscess.
Table 40.1
Literature on feasibility of radical pelvic resection (radical oophorectomy, modified posterior exenteration, or en bloc rectosigmoid colectomy)
Study | Year | Patients | Complications (%) | ||
|---|---|---|---|---|---|
Overall | Procedure-related | Mortality | |||
Soper et al. [18] | 1991 | 21 | NA | NA | NA |
Scarabelli et al. [19] | 2000 | 66 | 13 (20) | 1 (1) | 0 |
Obermair et al. [20] | 2001 | 65 | 19 (29) | 3 (5) | 1 (1) |
Clayton et al. [21] | 2002 | 129 | 38 (30) | 3 (2) | 4 (3) |
Bristow et al. [22] | 2003 | 31 | 4 (13) | 1 (3) | 0 |
Mourton et al. [23] | 2005 | 70 | 22 (31) | 4 (6) | 1 (1) |
Aletti et al. [16] | 2006 | 57 | NA | 1 (2) | 0 |
Park et al. [24] | 2006 | 46 | 15 (33) | 2 (4) | 0 |
Houvenaeghel [25] | 2009 | 168 | 45 (27) | NA | NA |
Tixier et al. [26] | 2010 | 41 | 14 (34) | 4 (10) | 3 (7) |
Extrapelvic Bowel Resection
Outside of the pelvis, the two most common sites of bowel involvement are the ileocecum and the transverse colon. Anatomic considerations when considering resection in these areas are primarily due to vascular supply. Variability in watershed areas, and the degree of communication between the ileocolic, right colic, and middle colic arteries need to be considered so that resultant anastomoses have adequate supply.
Perhaps because of this, or because patients with extrapelvic bowel involvement have more extensive disease, the reported procedure-related complication rate of approximately 8 % is slightly higher than after en bloc pelvic resection (see Table 40.2) [27–34]. In Hoffman’s series of 144 patients with advanced ovarian cancer, 36 % had extensive bowel involvement outside the pelvis, with a 6 % risk of procedure-related complication [28]. Silver and colleagues reported their series of 19 patients undergoing extended left colon resection, with a low overall complication rate and detailed analysis of relevant vascular considerations when rotating the remaining right colon around the ileocolic artery pedicle [33].
Table 40.2
Literature on feasibility of bowel resection
Study | Year | Patients | Complications (%) | ||
|---|---|---|---|---|---|
Overall | Procedure-related | Mortality | |||
Gillette-Cloven et al. [27] | 2001 | 105 | 18 (17) | 10 (10) | 6 (6) |
Hoffman et al. [28] | 2005 | 144 | 23 (16) | 9 (6) | 0 |
Estes et al. [29] | 2006 | 48 | 5 (10) | 4 (8) | 2 (4) |
Bidzinski et al. [30] | 2007 | 39 | 8 (20) | 5 (13) | 0 |
Salani et al. [31] | 2007 | 125 | 30 (24) | 11 (9) | 2 (2) |
Bristow et al. [32] | 2008 | 33 | NA | NA | 1 (3) |
Silver [33] | 2009 | 19 | 3 (16) | NA | 1 (5) |
Song et al. [34] | 2009 | 22 | 7 (32) | 0 | 0 |
Right Upper Quadrant Resection
The right upper quadrant is frequently involved due to the pooling of ascites containing metastatic cells in the right subphrenic space; this pooling is caused by gravity and the falciform ligament. Up to 40 % of women with advanced ovarian cancer present with bulky metastatic disease to the diaphragm, and diaphragm implants have been described as one of the most common factors precluding optimal cytoreduction [35]. The ability to safely remove these diaphragm lesions is an essential component of the comprehensive approach to surgical cytoreduction, which has been associated with improved survival in these patients [15]. The extent of resection required is determined by the surface area involved and the presence of muscular invasion. Primary anatomic considerations include the relevant hepatic attachments and the underlying central vasculature, as the right liver must be mobilized medially in order to gain access to the entire right diaphragm [36]. The coronary ligaments reflect off the liver capsule and delineate the posterior extent of the diaphragm peritoneum. The right hepatic vein drains into the inferior vena cava at the medial portion of the right coronary ligament, and additional caution is essential during this part of the dissection to avoid injury to this vessel. In addition, the right phrenic nerve penetrates the central tendon of the diaphragm but is usually not encountered until the right coronary ligament is divided and the base area of the liver is exposed.
Diaphragm peritonectomy and/or resection (Figs. 40.4 and 40.5) are generally well tolerated, and the reported complication rates vary somewhat between series, as shown in Table 40.3 [37–44]. This variability appears to depend on how pleural effusions were scored as complications, and whether a chest tube was placed as a prophylactic measure at the time of surgery. One series evaluating 59 patients in whom intraoperative chest tubes were not placed after diaphragm surgery and who had daily postoperative chest imaging showed a 58 % incidence of ipsilateral effusions; 15 % required postoperative drainage [42]. Chereau and colleagues recently reported their series in which 144 patients underwent diaphragm surgery for primary, interval, or recurrent disease; 35 % of the patients had chest tubes placed intraoperatively and 43 % developed pulmonary complications [44].
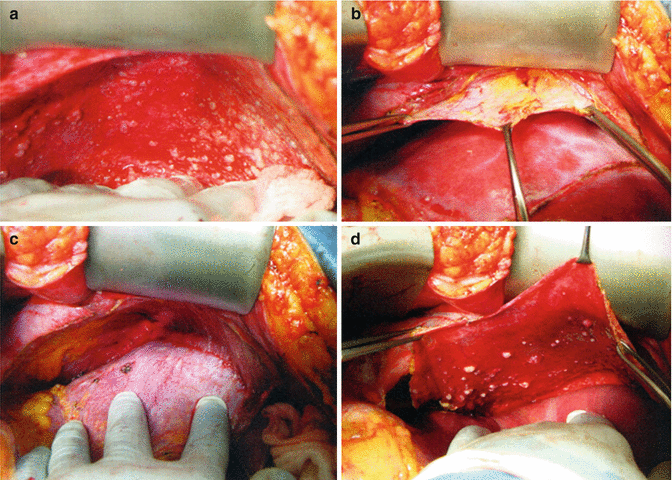
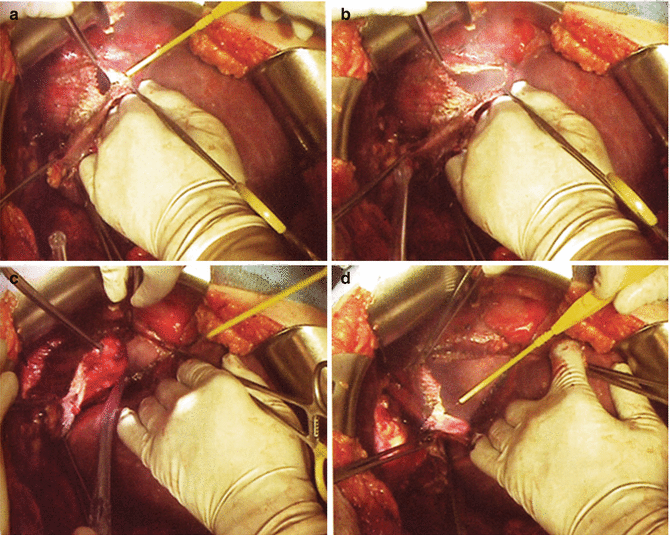

Fig. 40.4
Diaphragm peritonectomy. (a) The self-retaining retractor has been positioned to provide maximal elevation of the costal margin. (b) The diaphragm peritoneum is incised along the costal margin, developing a broad front of dissection in the subperitoneal plane. (c) The diaphragm peritoneum is placed on downward traction, exposing the plane of dissection at the interface with the muscular surface. (d) The dissection is carried posteriorly to the peritoneal reflection of the coronary and right triangular ligaments. (Reprinted with permission from: Bristow et al. [55])

Fig. 40.5
Full-thickness resection of the diaphragm. (a) The diaphragm muscle is incised with electrocautery and the pleural space entered. (b) The pleural space is explored to define the extent of resection. (c) The resection is carried posterior and laterally along the upper margin of the bare area of the liver. (d) The specimen is everted and the diaphragmatic pleura and muscle resected en bloc. (Reprinted with permission from: Bristow et al. [55])
Table 40.3
Literature on feasibility of right upper quadrant resection
Study | Year | Patients | Complications (%) | ||
|---|---|---|---|---|---|
Overall | Procedure-related | Mortality | |||
Diaphragm peritonectomy, resection or ablation | |||||
Montz et al. [37] | 1989 | 14 | NA | 1 (7) | 0 |
Silver et al. [38] | 2004 | 7 | 0 | 0 | 0 |
Cliby et al. [39] | 2004 | 41 | 8 (20) | 6 (15) | 0 |
Eisenhauer et al. [42] | 2006 | 59 | NA | 9 (15) | 0 |
Chereau et al. [40] | 2009 | 18 | 5 (28) | 4 (22) | 0 |
Einekel et al. [41] | 2009 | 30 | 14 (47) | 11 (37) | 1 (3) |
Gouy et al. [43] | 2010 | 63 | 11 (17) | 6 (10) | 0 |
Chereau et al. [44] | 2011 | 144 | 99 (69) | 62 (43) | 2 (3) |
Celiac axis or porta hepatis resection | |||||
Song et al. [45] | 2011 | 2 | 0 | 0 | 0 |
Martinez et al. [46] | 2011 | 28 | 10 (36) | 1 (3) | 0 |
Resection of metastatic disease from the celiac lymph nodes or porta hepatis can be difficult due to anatomic variations in the vascular anatomy and proximity to the hepatic and gastric vessels. Lymphadenectomy in this region is performed by surgical oncologists and hepatobiliary surgeons for staging or resection of some gastric, biliary, or hepatic cancers. Anatomic considerations in this region are multiple, including the celiac axis, portal triad, pancreas, and duodenum. Two recent series report successful ovarian cancer cytoreduction in this region with a low complication rate when performed with a multidisciplinary team including surgical oncologists, as shown in Table 40.3 [45, 46].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








