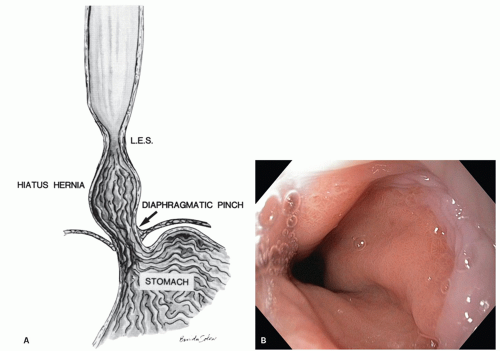
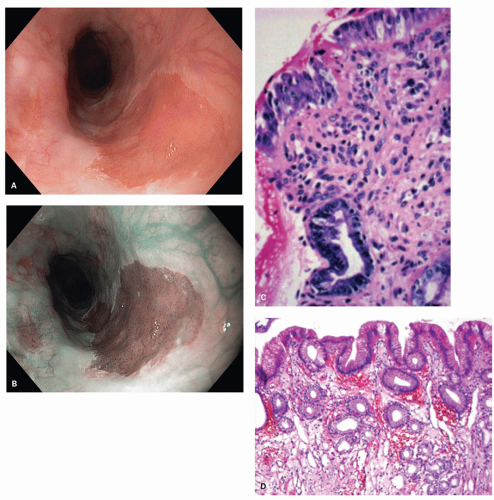
junction. This is reflected in the endoscopic appearance of the Z-line. Gastric mucosa may thus normally extend into the esophagus in this irregular (Z-line) fashion. Endoscopically the junction of tubular esophagus with the saccular stomach is defined by the upper border of convergence of the gastric folds, as neither the squamocolumnar junction nor the lower sphincter defined by manometry is considered reliable or accurate.
epithelium of skin. With electron microscopy the cell layers have been characterized as basal, prickle, and functional, the last referring to the flattened surface cells.2 The epithelium is generally 15 cell layer thick (350-450 µm). In clinical practice, one need to only distinguish between the basal and overlying nonbasal cells. The basal cell zone is one to four cells thick and does not exceed 15% of the thickness of the squamous layer except in the most distal centimeters of the esophagus.3, 4 The cells above the basal zone contain flattened nuclei and a glycogen-rich cytoplasm. The cells at the surface are elongated, with a very narrow margin of cytoplasm surrounding pyknotic-appearing nuclei. Basal cells are distinguished from nonbasal cells by the fact that the former do not contain glycogen. In clinical practice, using conventionally stained sections, basal cells appear juxtaposed. One rule of thumb used to define the upper margin of the basal layer is to determine where nuclei are separated by a distance of at least one nuclear diameter.5 Sometimes the tongues of columnar mucosa of the gastric type normally extend several centimeters on either side of the apparent LES zone. In addition, glands similar to those of the gastric cardia, with mucous glands and scattered fundic gland elements, commonly extend up into the lamina propria of the esophagus for a short distance.
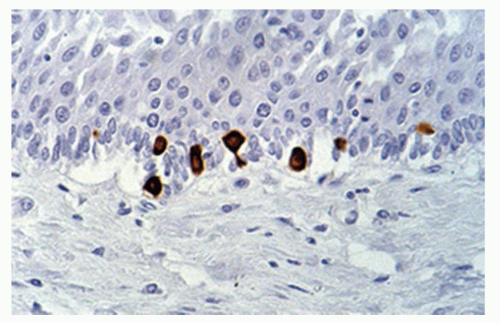
number seems to increase in esophagitis. Merkel cells are also found in the esophageal mucosa.9 Compared to fetuses their numbers are slightly increased in adults (1.2/cm vs. 2.2/cm). The numbers of Merkel cells vary markedly among individuals (Fig. 9-4). Immunohistochemically Merkel cells are positive for CK20. Merkel cells with their long dendritic processes can be identified from the 13th week of gestation onward. They are associated with light touch and in the skin can discriminate shape and textures, but it is unclear if they can do this in the esophagus.
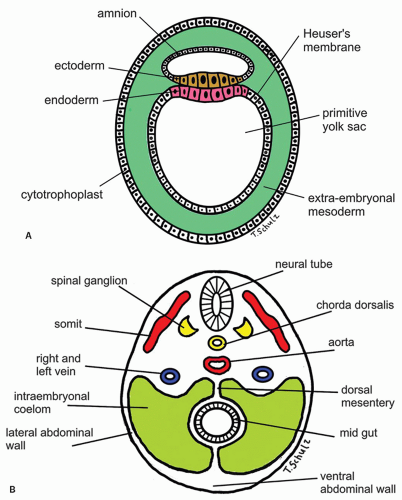
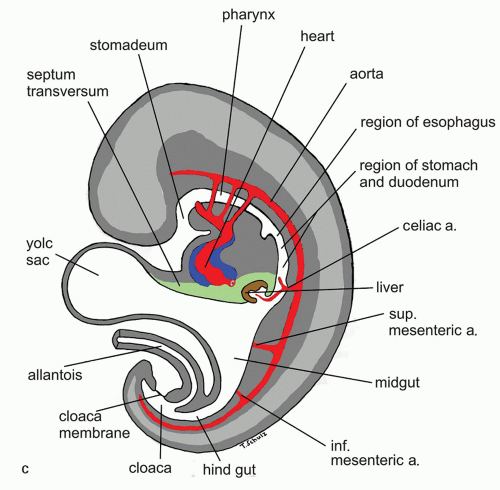 Figure 9-6. (Continued) C: Saggital cut of the embryo at 5th week of gestation showing various developing vascular structures and their relationship to the gut. |
can also be identified by the 7th week.25 From animal experiments it is known that interstitial cells of Cajal in the neural plexus and the circular muscle build networks for neural motor function. At time of birth it is concluded that neural networks are more mature in esophagus and stomach. Esophageal development of nerve and Cajal cell activity precede that in the remaining gut but have not achieved adult organization at time of birth. The timepoint of migration of Cajal cells into esophageal tissue is not yet known in detail. These findings explain that gut motility at birth does not show an adult pattern.32 The smooth muscle of the lower esophagus and the LES is derived from the mesenchyme of the somites surrounding the foregut. The striated muscle in the muscularis propria of the upper part of the esophagus and the upper esophageal sphincter is derived from mesenchyme of the fourth, fifth, and sixth branchial arches. This explains the innervation of the upper esophageal sphincter by the vagal nerve (the nerve of fifth branchial arch) and by the recurrent laryngeal nerve (a branch of the vagus nerve and the nerve of sixth branchial arch). The embryologic origin of the gastroesophageal junction is still controversial, but gastric rotation, together with augmentation of the fundus of the stomach, is believed to determine its formation.
recognized for the first time during adulthood due to recurrent respiratory infections.36
Table 9-1 Concomitant Anomalies in Individuals with Esophageal Atresia | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
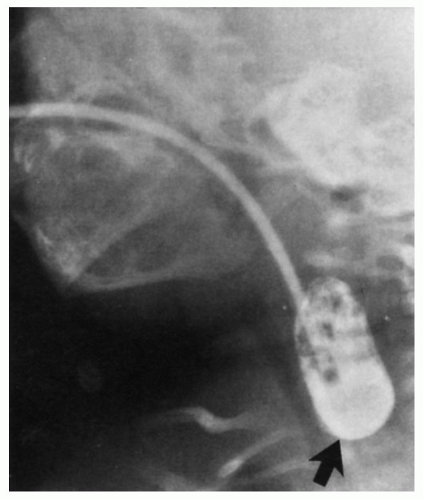 Figure 9-8. Esophageal atresia. Contrast placed via a tube fills the blind upper end of the esophagus. In this patient there was a distal tracheoesophageal fistula; see Figure 9-7, panel 1. (Courtesy of Marvin Weiner, M.D.) |
connected to the right main bronchus.38 The fistulous tract may be lined by a squamous, respiratory, or a transitional-type epithelium.39 Grossly several types of bronchoesophageal fistulas can be identified:
Table 9-2 The Different Types of Esophageal Atresia | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
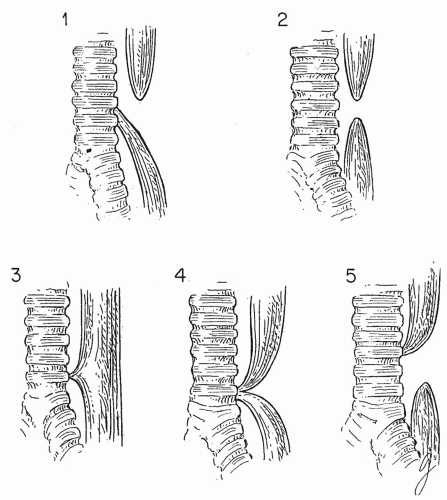 Figure 9-9. Various types of congenital tracheoesophageal fistulas and atresias. Approximately 85% are of the type illustrated in panel 1. Those represented in panels 4 and 5 are rare. |
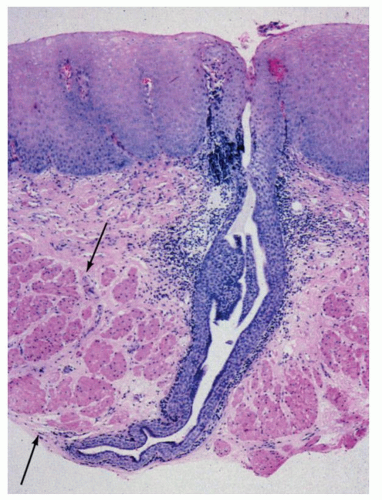
or mature type typical for that site). These need not communicate with the lumen. The esophageal duplications can become cystic, and it then becomes difficult to differentiate them from other congenital cysts (see later), and currently there are no reliable criteria to differentiate between these. Most would agree that an esophageal duplication should be contiguous with esophagus and have smooth muscle layers (Figs. 9-10 and 9-11). Most often these cysts are not connected to the esophageal lumen and are partially intramural or attached to the wall. Those that are connected to the esophageal lumen could be considered as esophageal diverticula.41, 42, 43 The lesions may become cystic or remain tubular. Histologically duplications show all the layers of the esophageal wall that include mucosa, muscularis mucosae, submucosa, and muscularis propria.44 The lining epithelium could be nonkeratinizing squamous epithelium, pseudostratified ciliated respiratory epithelium, or simple flat cuboidal to columnar epithelium. Rarely gastric fundic-type mucosa may be present, and some cases fail to show any lining epithelium at all. Many of the cysts defy classification and show a variety of lining epithelium and a fibrous wall. Depending on the appearance and the lining epithelium, a variety of names are in use. Some that are lined by gastric45 or small bowel-type epithelium46 have also been described that are referred to as gastroenteric cysts, while others are simply referred to as congenital esophageal cysts. The congenital cysts have a predilection for the lower third of the esophagus and rarely involve the upper esophagus.47
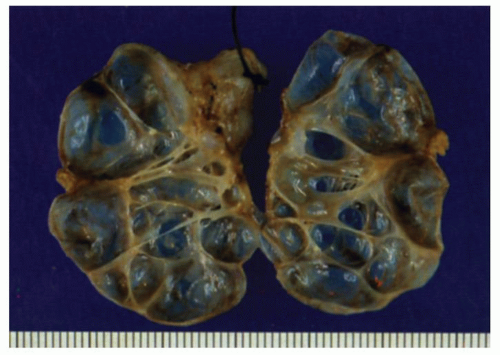
 Figure 9-11. Gross appearance of a Y-shaped communicating duplication of the esophagus. A portion of the stomach is shown at the bottom of the picture. |
acid, it is not surprising that they are rarely associated with burning sensations in the neck or with ulceration or cause strictures with resultant dysphagia.54, 55, 56, 57 Adenocarcinoma may arise in these ectopic patches but is rare.58 Some carcinomas have been described as arising from heterotopic gastric patches further downstream in the esophagus.58, 59 When inlet patches are encountered, there is no indication for biopsy. However, it is difficult for many endoscopists to resist the urge to biopsy the first few they encounter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree