Fig. 1
Prague C and M criteria. Image demonstrates the gastroesophageal (GE) junction, Prague C and M measurements in BE
Role of Endoscopy in the Management of Barrett’s Esophagus
Surveillance Endoscopy
Most published guidelines recommend endoscopic surveillance of BE. Surveillance for Barrett’s is recommended on two grounds. One, to detect EAC in an early and curable stage; two, to improve overall mortality in EAC and BE patients. Although some studies have suggested that there is some benefit with surveillance; these are limited by lead-time and selection bias [55, 56]. Further, mortality among patients with Barrett’s is equal to that of general population and most of them die from causes unrelated to EAC [57–59]. Presence of dysplasia requires confirmation with a second expert pathologist. Patients with no dysplasia undergo surveillance every 3–5 years, those with LGD every 6–12 months and those with HGD every 3 months if they do not undergo treatment (Fig. 2) [60–63].
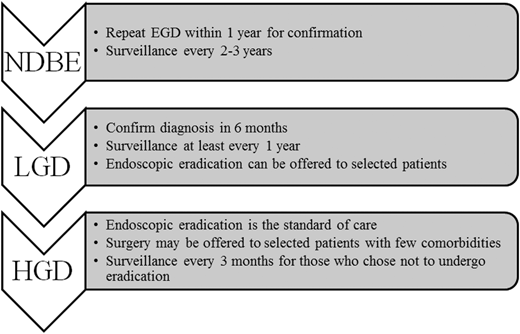

Fig. 2
Management algorithm for Barrett’s esophagus
Advanced Imaging in Neoplasia Detection
High Definition White Light Endoscopy
Compared to the standard low-resolution endoscopes that produce images of 300,000 pixels, high definition white light endoscopy (HD-WLE) can produce images with 1 million pixels. They help in detecting the subtle mucosal and vascular changes that are not otherwise seen with standard endoscopes. They have now replaced low-resolution endoscopes as the standard of care. In a prospective multi-center study using a HD-WLE endoscope, inspecting the BE segment for longer time was associated with the increased detection of lesions suspicious for HGD/EAC [64]. Barrett’s inspection time per centimeter correlated with detection of HGD and endoscopists with Barrett’s inspection time (BIT) greater than 1 min/cm were more likely to detect visible lesions. HD-WLE alone has been shown to have a sensitivity of 79–85 % and is best used with another advanced imaging modality [65, 66].
Chromoendoscopy
Chromoendoscopy utilizes stains to visualize mucosal abnormalities that are not readily apparent. Lugol’s iodine, acetic acid, and methylene blue have all been used as stains. Among these, methylene blue chromoendoscopy has been the most extensively studied. Light to absent staining and moderate to marked heterogeneity are associated with the presence of dysplasia [67]. While methylene-blue-directed biopsies were found to be very sensitive for the detection of intestinal metaplasia , they did not improve overall yield of dysplasia or cancer [68–73]. Methylene-blue-directed biopsies were also found to have fewer yields when compared to random biopsies using Seattle protocol [74, 75].
Narrow-Band Imaging
Endoscopes that use narrow-band imaging (NBI) filter blue light corresponding to the absorption peaks of hemoglobin making the vascular pattern stand out in contrast to the mucosal pattern. NBI filters reflect light, and there is no need for adding dyes or intravenous contrasts, but the steep learning curve associated with it impedes widespread use. In a randomized crossover study, both HD-WLE and NBI detected the same number of patients with Intestinal metaplasia (IM), while NBI detected a greater number of patients with dysplasia and required a fewer number of biopsies [76, 77]. A pooled analysis of seven published studies showed that using NBI increased the yield of dysplasia detection in endoscopy by 34 % [77]. Overall, the NBI has a sensitivity and specificity of 96 and 94 % [78].
Autofluorescence Imaging
Certain endogenous molecules, such as collagen and elastin, known as fluorophores emit long-wavelength light when excited by shorter wavelength light. Autofluorescence imaging (AFI) utilizes this property and differentiates dysplastic epithelium from nondysplastic epithelium based on the differences in the intensities of their emitted light. The dysplastic epithelium is associated with decreased autofluorescence. Nondysplastic BE appears green while dysplastic BE appears blue or violet. Due to the range in color observed, standardized color scales have been used for the diagnosis [79]. It has been suggested that AFI be used as a broad field technique to mark areas that appear dysplastic. However, randomized crossover studies have shown a sensitivity of 40–60 % and a high rate of false positives [65, 80]. To overcome this limitation, endoscopic trimodal imaging was performed. With this procedure, the esophagus was imaged using HD-WLE, followed by AFI. Visible lesion and abnormal areas were then further characterized by NBI. While adding AFI to HD-WLE led to the detection of increased number of patients and areas with dysplasia, NBI reduced the overall false positive rate [81, 82]. Again, these results were not seen in general practice [83].
Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (CLE) is a technique that allows in vivo visualization of mucosa with up to 1000-fold magnification and to a depth of 250 µm. Intravenous sodium fluorescein is used as a contrast. It is available as a standalone endoscope (eCLE) or as a probe-based system (pCLE) that can be integrated into standard endoscopes. The eCLE endoscopes have a free working channel to take biopsies and the images produced are slightly higher in resolution, but the rate of acquiring images is slower. On the other hand, the pCLE system can be mounted on a regular endoscope and it produces more number of images, but the images produced are of lower resolution and the field of images produced is smaller. The prospective multi-center DONT BIOPCE trial demonstrated that pCLE was able to detect additional patients that were not detected by white light endoscopy or NBI [84]. The sensitivity of CLE ranges from 75–100 % and specificity from 83–98 % [85–89]. Using the Kansas city criteria for the diagnosis of dysplasia, after a short structured teaching session the combined accuracy of experts and nonexperts was 81 %, with a substantial inter-observer agreement (κ = 0.61) [90].
Optical Coherence Tomography
Optical coherence tomography (OCT) is a system similar to ultrasound, wherein the images are generated from magnitude of optical echoes detected from tissues. The advantage of OCT is, it provides real time images of esophagus up to a depth of 3 mm that would otherwise be undetectable by regular endoscopy (Fig. 3). The Nvision VLE™ imaging system (Nine Point Medical Inc., Cambridge, MA) uses an optical probe with a balloon at its distal end that is 25 mm wide and 6 cm long. The probe scans the portion of esophagus in contact with it over a period of 90 s and produces circumferential images with a resolution of 10 µm. An early pilot study demonstrated the feasibility of this technique [91]. A 1450 nm cautery marking laser light is integrated into the system, enabling visualization and marking of abnormal areas at the same time [92]. Diagnostic criteria for the detection of HGD/EAC have been shown to have a sensitivity of 83 % and specificity of 75 % [93].


Fig. 3
Optical coherence tomography demonstrating submucosal glands (arrows)
Investigational Imaging Techniques
Fluorescent tagged peptides and lectins are being used to mark dysplastic areas in the esophagus [94, 95]. Strum and colleagues used phage display technology to detect peptides that bind to EAC cells [94]. They isolated the peptide ASYNYDA that was found to have 5.3 times greater affinity for human H460 adenocarcinoma cells compared to non-neoplastic human Q-hTERT BE cells. The peptide was labeled with fluorophore FITC, and the fluorescent tagged peptide, now called ASY*-FITC was tested in ex-vivo specimens and human subjects. In resected esophageal specimens, the fluorescence intensities for HGD and EAC were found to be higher than NDBE and squamous epithelium (Fig. 4). The ASY*-FITC binding was tested in 25 subjects with the BE using confocal endomicroscopy. ASY*-FITC binding produced 3.8 times greater signal intensity for HGD and EAC compared to squamous epithelium and NDBE. A target-background (T/B) ratio was used to measure the intensity of fluorescence and a T/B ratio of 4.2 was found to have a sensitivity of 75 % and specificity of 97 % for the detection of neoplasia. Lectins are proteins that can bind to specific carbohydrate sequences. A progression from squamous epithelium to EAC is associated with the change in surface glycans. Using unsupervised clustering analysis, Bird-Lieberman et al. identified a group of lectins with high-binding affinity to squamous epithelium with progressively decreased binding to EAC [95]. One such lectin, the wheat germ agglutinin (WGA) was further tested in ex-vivo specimens. In the four resected esophagi, WGA fluorescence was associated with a degree of dysplasia with areas of HGD and EAC showing low WGA binding. The mean signal to background ratio for dysplasia was 5.2, compared to squamous epithelium enabling easy detection in the ex-vivo specimens.
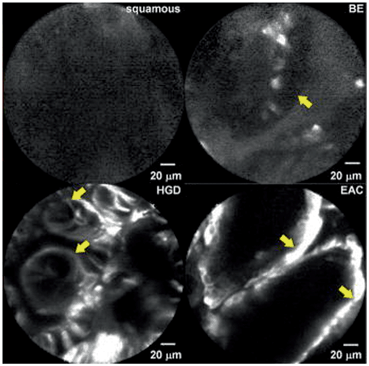

Fig. 4
Peptide-based imaging for Barrett’s esophagus. Fluorescent imaging of squamous, nondysplastic Barrett’s, high-grade dysplasia and esophageal adenocarcinoma
Light incident on tissue is scattered due to vibration of the molecules it is composed of and causes a shift in the frequency of the scattered light. The change in the frequency of light differs with the composition of tissue and this property it utilized by Raman spectroscopy to differentiate neoplastic from non-neoplastic BE. Using confocal Raman spectroscopy, Bergholt et al. were able to differentiate HGD from LGD and NDBE with sensitivity of 87.0 % (67/77), and a specificity of 84.7 % (610/720) [96]. The performance characteristics of these techniques need to be evaluated in large population-based cohorts.
Role of Endoscopic Eradication Therapy in Dysplastic Barrett’s Esophagus
Initially, the published surgical series reported rates of concomitant invasive cancer of 40 % [97–99]. However, these studies did not use standardized criteria for cancer. Using strict criteria and definitions, the rate of invasive cancer is now estimated to be 5.58 per 100 patient years [46]. This has shifted the treatment approach away from esophagectomy that is associated with high operative morbidity and mortality in favor of endoscopic eradication therapy . The endoscopic eradication therapy of Barrett’s is based on the premise that the removal of Barrett’s epithelium using resection or ablation in conjunction with acid suppression results in replacement of columnar by “new” or neo-squamous epithelium. Described below are various methods used in ablation of Barrett’s mucosa.
Multipolar Electrocoagulation
Multipolar electrocoagulation (MPEC) uses a thermal probe to ablate the squamous epithelium. The probe is placed in contact with the epithelium till the formation of white coagulum is observed. Multiple sessions are performed every 4–6 weeks till the entire Barrett’s mucosa is ablated. Studies have demonstrated a reversal of Barrett’s in 75–100 % of patients [100–103].
Argon Plasma Coagulation
Argon plasma coagulation (APC) is a thermal coagulation method used to ablate Barrett’s mucosa. The APC probe is passed down the biopsy channel and coagulation of the entire Barrett’s mucosa is attempted if the length of BE is less than 3 cm. For longer segments of BE, 50 % of circumferential mucosa is ablated to minimize the risk of stricture formation. Although complete ablation of Barrett’s is reported initially, long-term eradication has been reported in 39–88 % [100, 101, 104–109] with recurrence seen in up to 66 % [106].
Photodynamic Therapy
Photodynamic therapy uses drugs that, upon exposure to light react with oxygen and produce tissue necrosis. These drugs are called photosensitizers and several compounds such as porphyrins, chlorins, benzoporphyrins, and pheophorbides have been used. They differ on their excitation wavelengths, absorption, clearance and skin photosensitivity. The light source has to deliver light uniformly to the entire tissue, which is a challenge given the peristalsis—movements associated with breathing and esophageal folds.
Cryotherapy
Cryotherapy involves rapidly freezing and slow thawing of tissues resulting in vascular thrombosis and ischemia. The two available devices use liquid nitrogen and carbon dioxide. The cryotherapy probe can be passed through the working channel of the endoscope. Low pressure liquid nitrogen (< 5 psi) at -196°C, or high pressure CO2, (450–750 psi) is sprayed on the target tissue till a white frost is formed (Fig. 5) [110, 111]. The treatment is applied in doses ranging from two cycles of 20 s each to four cycles of 10 s. The treatment is repeated every 4–8 weeks till there is no evidence of residual BE [112, 113]. In a multi-center retrospective review, 98 patients with HGD were treated with cryotherapy for a mean 3.4 sessions per patient [114]. Sixteen patients completed the therapy, 58 (97 %) had complete eradication of HGD, 52 (87 %) had complete eradication of dysplasia and 34 (57 %) had complete eradication of all intestinal metaplasia after follow up of 10.5 months. Other studies have reported a complete eradication of dysplasia in 78–96 % of the cases [110, 112–116].
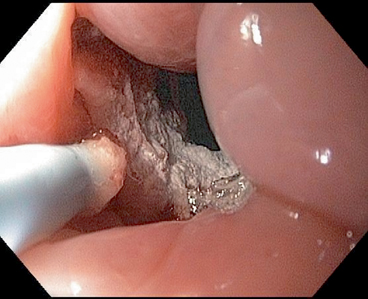

Fig. 5
Frost formed after cryotherapy
Radiofrequency Ablation
Radiofrequency energy can be delivered to the esophageal mucosa using a balloon that has electrodes mounted on it. A preset amount of energy can be delivered controlling the depth to which the tissue damage is limited. A soft sizing balloon is used to measure the diameter of the esophagus and a balloon device with a 3 cm mounted electrode is used to ablate 3 cm segments of BE. The circumferential ablation balloon (Barrx™ 360) is available in sizes of 18, 22, 25, 28, and 31 mm (Fig. 6). The procedure is repeated every 3 cm till the entire BE segment is ablated. A plastic cap at the end of facilitates cleaning of debris and tissue. Paddle-like electrodes that are mounted on the distal end of endoscope are available, if ablation of only a small region needs to be ablated. The Barrx™ 90 and 60 have electrodes sized 20 × 13 mm and 15 × 10 mm. An ultra-long (40 × 30 mm) catheter and a through-the-scope catheter 7.5 × 15.7 mm are also available.
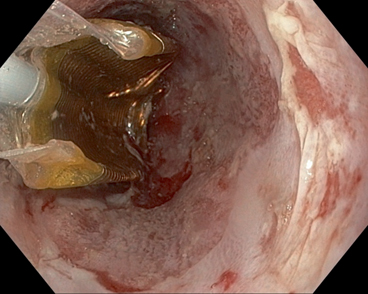

Fig. 6
Barrx™ 360 balloon (deflated) used for radiofrequency ablation in Barrett’s esophagus
The landmark Ablation of Intestinal Metaplasia (ATM) containing Dysplasia established radiofrequency ablation (RFA) as the preferred method of choice for eradicating dysplasia [117]. In this study, 127 patients with dysplasia were randomized to receive either RFA or sham therapy in a 2:1 ratio. Complete eradication of dysplasia was seen in 81 % of patients with HGD and 91 % of patients with LGD compared to 19 % and 22 % respectively seen in sham groups. At the end of 1-year follow-up of esophageal cancer developed in 19 % of the sham group compared to 1 % in the ablation group. After 3 years, dysplasia was eradicated in 98 % and intestinal metaplasia in 91 % of the patients [118]. Studies have reported rates of complete eradication of dysplasia ranging from 74–96 % and the median time to eradication to be 22 months [117, 119–126]. Predictors of poor response to RFA include active reflux esophagitis, regeneration of endoscopic resection with BE, esophageal narrowing before RFA and years of neoplasia before RFA. However, recent reports indicate recurrence rates of up to 33 % with dysplasia found in 22 % of those with recurrence [126, 127]. Factors such as longer BE segment, advanced age, and dysplasia have been found to be predictors of recurrence [126, 127]. Strictures seen in about 6 % of patients are the most common adverse event followed by pain (3 %) and bleeding (1 %) [126, 128].
Endoscopic Mucosal Resection
Endoscopic mucosal resection (EMR) is a snare-based technique that involves mucosal excision. It is used both for diagnostic and therapeutic purposes. EMR is better at diagnosing the depth of invasion than endoscopic ultrasound and has better inter-observer agreement among pathologists than biopsy specimens [129]. Although EMR is currently recommended for visible lesions, almost a third of patients with HGD have change in their diagnosis irrespective of whether visible lesions were present [130]. EMR of visible lesions followed by RFA of the rest of the Barrett’s epithelium used for resection led to the complete eradication of dysplasia in up to 100 % of the patients [121, 131]. Stepwise EMR can be done to completely eradicate dysplasia. In a prospective single-center study based in Germany, 349 patients with HGD were enrolled to undergo endoscopic resection [132]. Out of these, 337 (97 %) patients showed complete response and after a median follow up of 62 months, 330 (95 %) remained disease free. Other studies reported eradication rates of 97–100 % in patients undergoing this procedure [132–136]. Bleeding and perforation are seen in 1–3 % of patients and esophageal strictures are seen in up to 40 % of the patients .
Management of Nondysplastic Barrett’s Esophagus
All patients with nondysplastic BE are enrolled into a surveillance program with endoscopies performed every 2–3 years (Fig. 2). Lack of a clear evidence of benefit, low rates of progression, increased costs, recurrent disease, and risks associated with treatment argue against endoscopic eradication of BE in this subgroup.
Management of Low-Grade Dysplasia
The management of patients with low-grade dysplasia is complicated by several factors. There is a significant variability in the diagnosis of low-grade dysplasia, even among expert gastrointestinal pathologists. The progression to EAC is variable and there is no evidence to suggest that endoscopic eradication prevents or reduces progression to EAC. The long-term durability of endoscopic treatment is unknown with a recent radiofrequency ablation registry study demonstrating a recurrence of 28 % [127]. A cost-utility analysis demonstrated that endoscopic eradication can be cost-effective if ablation of continued surveillance were not necessary [137]. However, the SUrveillance vs. RadioFrequency ablation (SURF) study, a randomized controlled trial where patients with LGD confirmed by two expert pathologists underwent either RFA or surveillance, showed complete eradication of dysplasia or intestinal metaplasia in 98 % of patients in the RFA arm with decreased rates of progression in the RFA arm (1.5 % versus 20.6 %) [138]. Therefore, the treatment of LGD is a moving target at this stage. In the absence of long-term data, surveillance is recommended with ablation reserved as a therapeutic option to be discussed with the patients. Rigorous surveillance with four-quadrant biopsy must be performed in all patients to detect early HGD/EAC.
Management of High-Grade Dysplasia
Due to the high rates of progression and prevalent EAC [45, 46], endoscopic eradication is recommended for patients with high-grade dysplasia. The recommended approach in patients with high-grade dysplasia is the removal of visible lesions using endoscopic mucosal resection followed by radiofrequency ablation of all visible Barrett’s epithelium. Patients who have undergone eradication are enrolled into surveillance programs to detect recurrence of intestinal metaplasia or dysplasia. Due to the high efficacy of radiofrequency ablation, esophagectomy is now rarely used for patients with high-grade dysplasia. Most modern studies report an acceptable operative mortality of less than 5 % [139–146]. The advantages of esophagectomy include removal of the entire esophagus at the risk of developing recurrent dysplasia obviating the risk of sub-squamous metaplasia and removal of lymph nodes to which invasive cancer may have spread. This procedure must be therefore reserved for individuals with fewer comorbidities reducing the risk of perioperative mortality and complications.
Complications of Endoscopic Therapy
Strictures
This is the most common complication following endoscopic eradication therapy. Strictures are most commonly seen following EMR in 30–50 % of the patients [133–136]. The length of BE determines the risk of stricture formation; therefore, it is recommended that complete mucosal resection be reserved as a method for those with BE length less than C3 M5. Following RFA, strictures are seen in 5 % of the patients with higher rates reported in the series that used EMR in combination with RFA [118–124, 131]. Cryotherapy carries a 10 % risk of strictures while other modalities such as APC and MPEC carry a 2–3 % risk [114–116]. Esophageal strictures can easily be treated with balloon or bougie dilation .
Perforation and Bleeding
Perforation and bleeding are acute complications that are seen in the first 48 h. They are seen in about 1 % of patients undergoing EMR and less than 1 % of patients undergoing endoscopic therapies. Most of the patients with these complications can be managed appropriately. Death as a result of these or other endoscopic therapies is exceedingly rare.
Subsquamous Intestinal Metaplasia
Subsquamous intestinal metaplasia (SSIM) also known as buried Barrett’s is the presence of intestinal metaplasia under squamous epithelium. It was previously assumed that this represented residual Barrett’s epithelium over which squamous epithelium had grown following ablation. However, we now know that SSIM is present in patients before eradication. In the AIM dysplasia trial [117] and another study using EMR for eradication, they were found in a fourth of all patients [147]. However, since they are not detectable using most of the endoscopic techniques and due to the fact that our biopsies are inadequate in depth and orientation, we may be underestimating the true prevalence [148]. Recent reports have indicated that similar to surface metaplasia, SSIM has malignant potential and need to be carefully evaluated [149, 150]. 3D-optical-coherence tomography is one technique that allows visualization of esophageal mucosa up to a depth of 3 mm. Using this technique, one series demonstrated that SSIM can be seen in 72 % of the patients before and 63 % of the patients after ablation [151]. Further refinements in this technology as well as biopsy technique are necessary to explore this condition further.
Future Direction
Although endoscopic eradication has largely replaced surgical therapy for the treatment of HGD in BE, long-term data demonstrating efficacy at 5–10 years is lacking. We also do not know if endoscopic eradication will lead to reduction in the incidence of invasive cancer. Advanced imaging techniques such as fluorescent-tagged peptides and lectins need to be validated in large population-based cohorts. Biomarker panels are being developed to detect patients at the risk of progressing to EAC. Hypermethylation of CpG island promoter region has been shown to be associated with neoplastic progression in BE. Jin et al. performed a retrospective, double-blinded evaluation of methylation of eight genes (p16, HPP1, RUNX3, CDH13, TAC1, NELL1, AKAP12, and SST) to identify patients who progressed to dysplasia [152]. The combined eight-gene panel had an area under the receiver-operating curve (AUROC) of 0.840. Similarly, Avli et al. identified a hypermethylated four-gene panel (SLC22A18, PIGR, GJA12, and RIN2), and validated in both retrospective and prospective cohorts. The four-gene panel had an AUROC of 0.988 with a sensitivity of 94 % and specificity of 97 %. Deoxyribonucleic acid (DNA)-based fluorescent in situ hybridization (FISH) is also being used to predict progression of BE. Using FISH analysis of brush biopsy specimens from BE patients, Her2/Neu, c-Myc, and 20q13.2 overexpression and aneuploidy of chromosomes 7 and 17 were used to differentiate HGD/EAC patients from NDBE [153]. A combination of these biomarkers was found to have an AUROC of 0.90 with a sensitivity of 88 % and specificity of 100 %. These panels are yet to be incorporated into routine clinical use.
Finally, genome wide association studies have identified 5 SNP loci associated with BE and EAC at 19p13, 9q22.32, 3p13, 16q24 and 6p21. These studies indicate significant polygenic overlap between EAC and BE [154, 155]. Further research in this area will provide insight into the development of BE and EAC.
Summary
The BE is a premalignant condition of the esophagus that leads to the development of esophageal cancer. It is seen in older Caucasian males with a large waist to hip ratio who smoke. It progresses through stages of NDBE, LGD and HGD before developing into invasive cancer. Endoscopy is necessary for diagnosis, treatment, and surveillance of these patients. The most commonly used endoscopic eradication technique is EMR of visible lesions followed by RFA of the remaining BE. Current studies indicate a recurrence rate of 30 % for most eradication techniques and underscore the need for continued surveillance among these patients.
References
1.
Vakil, N, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–20 (quiz 1943).PubMed
2.
Sharma P, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127(1):310–30.PubMed
3.
Spechler SJ, et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):e18–52 (quiz e13).PubMedCentralPubMed
4.
Williamson WA, et al. Barrett’s esophagus. Prevalence and incidence of adenocarcinoma. Arch Intern Med. 1991;151(11):2212–6.PubMed
5.
Bosetti C, et al. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122(5):1118–29.PubMed
6.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.PubMed
7.
Zagari RM, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57(10):1354–9.PubMed
8.
Ronkainen J, et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129(6):1825–31.PubMed
9.
Hayeck TJ, et al. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23(6):451–7.PubMedCentralPubMed
10.
Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361(26):2548–56.PubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








