- Esophageal squamous cell carcinoma (ESCC) is still more prevalent than adenocarcinoma worldwide. In western countries, high-risk individuals include smokers, patients with head and neck squamous cell carcinoma, tylosis, achalasia, lichen planus, scleroderma, Plummer–Vinson syndrome, and prior radiation of neck and chest.
- Esophagogastroduodenoscopy (EGD) with Lugol spray is currently considered as the most effective noninvasive way to identify squamous cell dysplasia and ESCC. Newer modalities such as narrow band imaging (NBI), confocal laser endomicroscopy (CLE), and autofluorescence imaging (AFI) are promising.
- Treatment for ESCC is based on the stage of the disease. Currently, ESCC that is limited in the mucosa can be managed by endoscopic mucosal resection. Patients with more advanced disease are candidates for surgery with or without neoadjuvant/adjuvant chemotherapy if it is resectable. Otherwise, for unresectable lesions, standard supportive care with or without chemotherapy is reasonable.
- The best strategies to reduce the incidence and mortality of ESCC are primary prevention and early diagnosis.
US National Cancer Institute—a comprehensive database assessable to the public for various types of cancers
Cancer Care Ontario, Canada—updated practice guidelines for prevention and treatment of ESCC
US National Comprehensive Cancer Network—guidelines, education programs for heal care providers and patients
American Society of Clinical Oncology—practice guidelines, research resources, education and training, public policy
The Role of Endoscopy in the Assessment and Treatment of Esophageal Cancer
- Physicians should be vigilant in diagnosing ESCC particularly among the high-risk subgroups.
- Endoscopic mucosal resection (EMR) is the treatment of choice for ESCC that is T1a (limited to the mucosa) or less. Avoid excessive deep biopsies of these lesions so that EMR can be safely performed for diagnosis and potential cure.
- For more advanced ESCC lesions, coordinated care involving gastroenterologists, medical oncologists, and thoracic surgeons is essential to achieve the best clinical outcomes.
Epidemiology
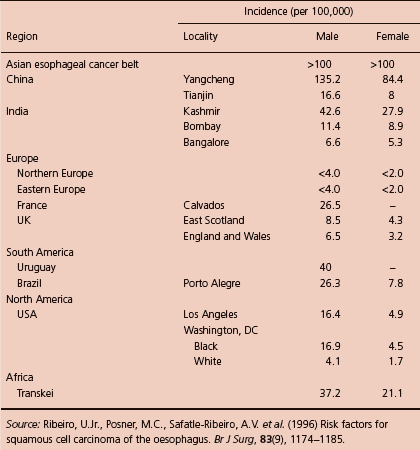
On a global basis, esophageal squamous cell carcinoma (ESCC) is the leading cancer of the esophagus, and it has been ranked as eighth in incidence and sixth in mortality among tumors of all sites.1 However, its incidence varies significantly among different geographic and ethnic subgroups (Table 1.1).2–6 The Asian Esophageal Cancer Belt, including western and northern China, Mongolia, southern parts of the former Soviet Union, Iran, Iraq, and eastern Turkey are considered the highest risk areas. The highest rate of incidence, 700 per 100,000, was reported in Linxian, China.3 The factors associated with esophageal cancer in these high-risk areas vary as to the population. It is interesting to note that protective factors that have been identified include increased consumption of fresh fruit and vegetables, eggs, meat, and central water supply. The risk factors for this high incidence are still to be further elucidated, but they likely include cigarette smoking, pipe smoking, excessive alcohol use, dietary habits (vitamin deficiency, etc.), differences in cooking, and environmental exposure. In Linxian, China, for example, high levels of polycyclic aromatic hydrocarbons have been found in the food that implicates cooking fuels as a potential source of this carcinogen in this high-risk area. It is important to note that risk factors such as human papilloma virus present in head and neck cancers do not seem to be a factor in squamous cell cancer in the esophagus.
Table 1.1 Incidence of esophageal squamous cancer in selected regions of the world.
The incidence of ESCC in the United States has been declining since 1973. This is in line with decrease of adult cigarette smoking rate from about 42% in 1960s to about 20% currently according to Centers for Disease Control and Prevention (CDC) reports. However, this decline of cigarette smoking has been stalled and caused significant public health concerns as the U.S. failed to drop below 12%, a goal set by Healthy People 2010. In addition, there appears to be an increase in younger smokers that may lead to a recurrence in the rate of cancer. Primary prevention of cancer is thought to be the most effective strategy in this disease in which the risk factors have been well established.
Although esophageal adenocarcinoma has surpassed ESCC since early 1990s in the United States, a high incidence is still seen among urban population and African Americans,1 and patients with certain comorbidities such as achalasia, head and neck cancer, and tylosis (Table 1.4). The incidence among African Americans men (16.8 per 100,000) was five times higher than Caucasians (3.0 per 100,000).4 And mortality was three times higher. In western countries, consumption of tobacco and alcohol could explain more than 90% of ESCC cases.4 The higher ESCC rate among African Americans parallels the adult cigarette smoking rates. According to Surveillance, Epidemiology, and End Results (SEER) cancer registry data from 1992 to 1998, ESCC incidence rates for Native American and white Hispanics were not higher than general population, although select Native American populations in specific regions of the country may have a higher incidence. Data from this group is influenced by the diversity of social and economic situations around the country.
Diagnosis
Patients with ESCC may present with dysphagia, weight loss, cough, and GI bleeding (hematemesis and/or melena). But there is no specific physical finding for ESCC, and rarely lymph nodes in the periphery could be appreciated. For cases with metastatic lesions, hepatomegaly could be present.
The following modalities are commonly used to establish the diagnosis of ESCC:
Lugol solution has been used in medicine since 1985. During EGD exam, Lugol solution, approximately 10–20 mL of 1.5% Lugol iodine solution (but the concentration may vary), is applied through a catheter over the entire esophagus. Since Lugol solution contains potassium and iodine, it should be avoided in patients with hyperthyroidism, iodine allergy, and renal insufficiency. Some authors believe that patients with hypopharyngeal tumors are not candidates for Lugol’s unless under endotracheal intubation due to concerns of possible laryngeal edema caused by iodine.
The Lugol staining pattern is associated with the degree of glycogen within the squamous epithelium, and squamous cell carcinoma does not include glycogen; hence, it is not stained and a clear identification is feasible. This enables endoscopist to visualize the dysplastic areas as Lugol-voiding lesions (LVLs). Biopsies could then target these LVLs to increase the yield. The overall sensitivity is 96–100% and specificity varies from 40% to 95%. It could also be used for intraoperative determination of tumor margins to assist surgical resection.
LVLs could also be of prognostic value. In a study of 227 patients with head and neck squamous cell carcinoma (HNSCC), those with no LVLs did not have metachronous ESCC during median follow-up of 28 months; however, 15% of those with numerous irregular LVLs lesions developed ESCC.7 One study examined nondysplasia epithelium (NDE) from LVLs, and it found 20% of them had a p53 hotspot mutation, and 40% among dysplasia epithelium in contrast to no p53 mutations in 103 paired NDE samples with normal Lugol staining. It was also suggested that the chance of finding dysplasia was much higher from a patient with more LVLs than those with fewer ones.
EGD with Lugol spray is currently considered as the most effective noninvasive way to diagnose squamous cell dysplasia and ESCC. Other newer methods such as narrow band imaging (NBI) or autofluorescence imaging (AFI) have been compared with Lugol spray to assess their accuracy. It is important to note that not all squamous cell cancers are Lugol voiding.
NBI is a novel noninvasive endoscopic approach to visualize the microvasculature on tissue surface. Compared with white light endoscope (WLE), NBI imaging uses blue light at 415 nm and green light at 540 nm, which gives hemoglobin special absorption characteristics. Thus, it provides better visualization of superficial and subsurface vessels that helps ESCC detection. Often times, the ESCC lesion appears reddish, likely due to microvascular proliferation and/or dilation.
In one nonrandomized study of HNSCC patients, NBI endoscope with magnification was proved to have very high sensitivity, specificity, accuracy, positive predictive value, and negative predictive value (100%, 97.5%, 97.8%, 83.3%, and 100%, respectively).8 In another multicenter, prospective, randomized controlled trial with 320 patients, NBI was shown to have 97% sensitivity for superficial ESCC.
As to high-grade dysplasia (HGD), one study showed the intraepithelial papillary capillary loop (IPCL) patterns were very helpful. But sensitivity and specificity were not satisfactory in contrast to a recent meta-analysis that demonstrated NBI was very sensitive (96%) and specific (94%) in detecting HGD and intramucosal adenocarcinoma for Barrett’s esophagus. It is noteworthy that all studies in this meta-analysis used NBI from a GIFQ240Z scope, an instrument that maintains the capabilities of a standard video endoscope and also affords a continuous range of image magnification adjustment up to X80.
However, NBI is not for detecting the depth of esophageal lesions based on current studies.
When white light from a xenon lamp travels through a special optical filter, only the blue excitation light at 390–470 nm and green reflected light at 540–560 nm penetrate through. Interestingly, the blue excitation light can cause living tissue to emit autofluorescence, which passes through another filter and then captured by the charged coupled device at the end of scope. AFI system works by combining autofluorescence (from blue light) and reflectance (from green light) to differentiate the neoplastic lesions (appears purple or magenta) from normal background (green). For the EGD scope that is equipped with AFI, the endoscopist can simply press the AFI button to switch from regular WLE to AFI. However, the flat or depressed ESCC lesions appear to be dark green, which makes it very difficult to distinguish the green color from normal squamous cell background.9 Because of this, AFI was considered not as sensitive as NBI for these flat or depressed lesions, making it a less attractive method despite that AFI had higher ESCC detection rate (79%) compared with WLE (51%). A multicenter randomized trial showed that in detecting dysplasia and early cancer from Barrett’s mucosa, the sensitivity, specificity, positive predictive value, and negative predictive value for AFI were 42%, 92%, 12%, 98.5%, respectively. Thus, at current time, AFI is best used as a complimentary method and not a screening test due to the low sensitivity.
AFI has also been used in bronchoscopy and colonoscopy for squamous cell carcinoma of lungs and dysplasia among ulcerative colitis patients in some studies with various results.
CLE is a new technology that allows in vivo examination of histopathology at the cellular and subcellular levels by using cellular and vascular criteria. The term “confocal” refers to the alignment of both illumination and collection systems in the same focal plane. The laser light could be focused at the different layers of the tissue of interest. Then the reflected light from this layer is refocused and allowed to pass back to the lens in endoscope and to be processed and presented on the monitor. Thus, different depths of tissue can be examined in vivo, the so-called optical biopsy. Fluorescent contrasts, either intravenously or sprayed topically, can enhance the quality of CLE imaging.10
In a recent study, CLE provided an in vivo diagnosis in 21 patients who had known ESCC, and the sensitivity and specificity using histology as gold standard were 100% and 95%, respectively. It holds promise for determination of the depth of squamous cell esophageal cancer.11 Another CLE study after Lugol spray and intravenous fluorescein sodium showed that the overall accuracy was 95%, and sensitivity and specificity were 100% and 87%, respectively. Intraobserver agreement was almost perfect (kappa, 0.95) and interobserver agreement was substantial (kappa, 0.79).12
CLE would potentially enable the endoscopist to proceed directly to endoscopic therapy, saving time and avoiding expensive and unnecessary further endoscopies. However, due to the limited tissue infiltration from the blue laser light, CLE may not be the right choice for submucosal lesions.
After systematic metastatic lesions are ruled out for ESCC patients, EUS could be performed by using either conventional EUS scope or miniprobe sonography (MPS) through the regular endoscope channel. It is considered as the most accurate noninvasive method for T staging and evaluation of lymph nodes around esophagus. It could also evaluate other organs such as adrenal glands, pancreas, liver, bile ducts, and mediastinal structures. Fine needle biopsy of lymph nodes can be done if necessary. However, it is difficult to distinguish T1a and T1b lesions sometime even with MPS. When a patient has scarring from previous radiation therapy (RT), endoscopic resection, or significant ongoing inflammation, it is also very challenging to provide accurate information. Despite of all these, the overall T staging accuracy of EUS is 85–90% as compared with 50–80% for CT; the accuracy of regional lymph nodes staging is 70–80% for EUS and 50–70% for CT. However, a recent review showed that T-stage from EUS had concordance of only 65% when compared with pathology specimens obtained by endoscopic mucosal resection (EMR) or surgery.
MPS is a small probe that could safely pass through a tight stricture or narrowing, and it could achieve higher resolution by using higher frequency. The use of MPS can also represent an improvement in the comfort and safety and is highly cost-effective.13 The drawbacks for MPS are (1) unable to perform real-time ultrasound-controlled fine needle aspiration and (2) lower penetration depth due to higher frequency used, which means less satisfaction in assessing structures (lymph nodes, etc.) that are further away from GI tract.
An esophagram with barium may identify a mass lesion. However, this role has been largely supplanted by EGD exam, which could in addition provide biopsy of suspected tissues. Once HGD or mucosal ESCC are identified, chest CT with or without PET scan should be used to assess systemic involvement. This global evaluation of a patient’s metastatic status (M and N staging) should be carried out before EUS.
For T staging, EUS is certainly superior to PET scan, which can only be considered when EUS or CT is inadequate. For N staging, EUS could more reliably distinguish the primary tumor from periesophageal lymph nodes based on a review in 2007. In centers with adequate experience, EUS should be the first choice unless it can not be performed due to stenosis. For M staging, PET scan has clear advantage for detection of disease beyond the celiac axis; however, it is challenging to differentiate the regional node, N1 node, and the celiac axis M1a node. As to the overall impact on the management, PET scan changed 17% of patients from curative to palliative, 4% from palliative to curative, and another 17% changed in treatment modality or delivery based on the results from a study with 68 esophageal cancer patients.
United States Preventive Services Task Force (USPSTF) recommends PET scan to improve the accuracy of M staging for patients who are potential candidates for curative therapy; however, no adequate research examined the value to predict response to neoadjuvant therapy or recurrence.
Recently, the accuracy of diffusion-weighted MR imaging for postoperative nodal recurrence of ESCC was found comparable with FDG-PET. The role of MRI certainly needs more studies to be further defined.
Some surgical centers use these methods for esophageal cancer staging because of the superiority over noninvasive methods. Indeed, an intergroup trial of 107 patients reported that thoracoscopy and laparoscopy could increase the detection rate of positive lymph node from 41% when using noninvasive staging tests (e.g., CT, MRI, EUS) to 56% by thoracoscopy and laparoscopy, and no major complications or deaths were reported. A more recent study in 2002 examined 111 esophageal cancer patients and compared thoracoscopy and laparoscopy versus noninvasive methods such as CT, MRI, and/or EUS, and it showed very low concordance ranging from 14% to 25% for TMN staging. This study pointed out that when compared with the final surgical pathology, a 100% specificity and positive predictive value was achieved by thoracoscopy and laparoscopy staging in diagnosis of lymph node metastasis. Although the sensitivity was about 75% (vs. 45% from noninvasive tests), the accuracy of thoracoscopy and laparoscopy could reach 90.8% and 96.4% in chest and abdomen metastases, respectively; these values were significantly higher than noninvasive staging methods (58% and 68%, respectively, for chest and abdomen).
Staging
The typical workup includes CT scan of chest (and abdomen if advanced lesions are suspected), PET (integrated PET–CT is preferred), and EUS if no metastatic lesions are found, and then surgical consult should be offered if it is resectable. The American Joint Committee on Cancer (AJCC) recently released its 7th edition of cancer staging manual (Table 1.2; Figure 1.1).
Table 1.2 TNM staging of esophageal squamous cell carcinoma (ESCC).
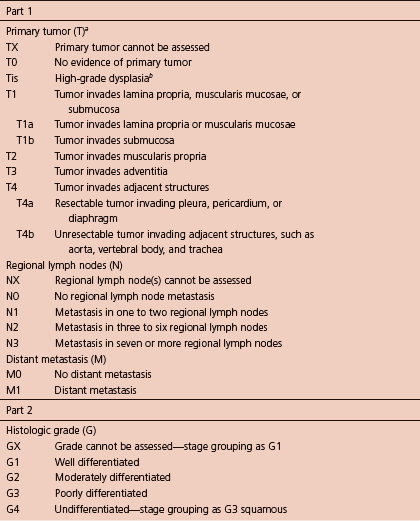
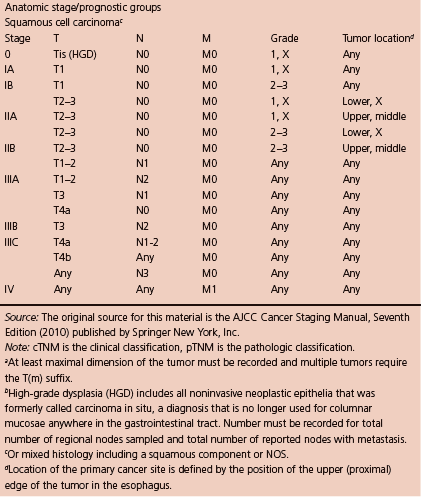
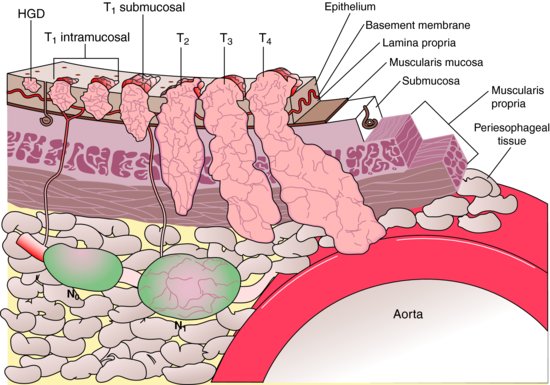
Figure 1.1 Layers of esophagus and stages of esophageal cancer. (Reproduced from Rice, W.R. (2002) Diagnosis and staging of esophageal carcinoma. In: Pearson, F.G., Cooper, J.D., Deslauriers, J. et al. (eds), Esophageal Surgery, 2nd ed, p. 687. Churchill Livingstone, New York.)
Prognostication
The most significant prognostic factor is TMN staging, although emerging biomarkers could also explain some of the variations in survival:
An early study in 1990s showed that early ESCC lesions that did not invade through muscularis mucosa had low lymph node metastasis rate (2–4%) or vascular invasion (8%).14,15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








