Esophageal malignancy is a major source of morbidity and mortality, despite the recently increased attention to screening and early detection. Prognosis for esophageal cancer remains grim, with advanced tumor stage and lymph node metastases conferring even graver outcomes. Several studies have demonstrated that the addition of preoperative neoadjuvant chemoradiotherapy may improve survival in patients with locally advanced tumor (T3) disease or local lymph node metastases. It is here that endoscopic ultrasonography finds its niche in the precise staging of these tumors and the subsequent use of stage-dependent treatment protocols.
Esophageal malignancy (primarily squamous and adenocarcinoma) is a major source of morbidity and mortality, despite the recently increased attention to screening and early detection. It remains as the seventh leading cause of cancer death worldwide, with a reported incidence as high as 30 to 800 cases per 100,000 persons in particular areas of northern Iran, southern Russia, and northern China. The worldwide epidemiology differs from that in the United States, with squamous cell carcinoma responsible for approximately 95% of all esophageal cancers. The European-weighted survival, calculated from the pool of all cancer registries, was 33% at 1 year and 10% at 5 years. The 3-year survival rate of patients with loco-regional spread after curative resection still remains low. In the United States in 2008, the American Cancer Society estimates that there will be 16,470 new cases (12,970 men and 3,500 women) of esophageal cancer diagnosed, and 14,280 persons (11,250 men and 3,030 women) are expected to die of the disease. Adenocarcinoma, as opposed to its worldwide squamous counterpart, has the fastest growing incidence rate of all cancers in the United States, with an age-adjusted incidence of 5.8 cases per 100,000 persons.
Prognosis for esophageal cancer still remains grim, with advanced tumor stage and lymph node metastases conferring even graver outcomes. The TNM (Tumor, Lymph node and Metastasis) staging of esophageal cancer has undergone many refinements over the course of the past few years ( Box 1 and Table 1 ). Several studies have demonstrated that the addition of preoperative neoadjuvant chemoradiotherapy may improve survival in patients with locally advanced tumor (T3, -4) disease or local lymph node metastases. It is here that endoscopic ultrasonography (EUS) finds its niche in the precise staging of these tumors and the subsequent use of stage-dependent treatment protocols.
TX–Tumor cannot be assessed
T0–No evidence of primary tumor
Tis–Carcinoma in situ
T1–Tumor invades submucosa or lamina propria
T1a–Tumor invades lamina propria
T1b–Tumor invades submucosa
T2–Tumor invades muscularis propria
T3–Tumor invades adventitia
T4–Tumor invades adjacent structures
NX–Regional lymph nodes cannot be assessed
N0–No regional lymph nodes involved
N1–Regional lymph nodes present
MX–Distant metastasis cannot be assessed
M0–No distant metastasis
M1a–Metastasis in the celiac lymph nodes if the tumor is in the lower one-third of the esophagus
M1a–Metastasis in the cervical lymph nodes if the tumor is in the upper one-third of the esophagus
M1b–Nonregional lymph nodes or other distant metastasis
From Catalano MF, Sivak MV Jr., Rice T, et al. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994;40:442–6.
| TNM | Stage | ||
|---|---|---|---|
| Tis | N0 | M0 | 0 |
| T1 | N0 | M0 | 1 |
| T2 | N0 | M0 | IIA |
| T3 | N0 | M0 | |
| T1 | N1 | M0 | IIB |
| T2 | N1 | M0 | |
| T3 | N1 | M0 | III |
| T4 | Any N | M0 | IV |
| Any T | Any N | M1 | |
EUS staging
EUS employs the technology of endoscopy coupled with internally placed high-frequency ultrasound waves. An ultrasound transducer mounted on the tip of the endoscope permits accurate imaging of tumors and lesions located within and adjacent to the esophagus. In addition, EUS-guided fine-needle aspiration (EUS-FNA) may be performed in adjunct to both confirm tumor diagnosis and facilitate staging.
Conventional EUS has two designs: the radial scanner and the linear scanner. The 360° radial scanner produces a 360° view perpendicular to the shaft of the endoscope and has the option of scanning at 12 MHz or 7.5 MHz. The higher frequency (12 MHz) permits better visualization of details at close range; the lower frequency (7.5 MHz) serves the purpose of better ultrasound wave penetration, thus permitting a greater evaluation field. This radial echoendoscope is useful because it gives a 360° overview, similar to a computer tomogram, allowing complete visualization of the gastrointestinal tract and its adjacent structures.
The linear array sector scanner operates at 5 MHz or 7.5 MHz and has color Doppler capability useful in the imaging of vascular structures. Ultrasound scans are made parallel to the shaft of the scope and has a limited (100°) sector of scanning area. The curved linear-array design permits direct needle aspiration (FNA). These sector scanners are preferred for use in EUS-FNA because they allow direct visualization of the needle up to 5 cm to 6 cm parallel to the shaft of the endoscope.
EUS has the unique ability to image the esophageal wall as a series of definable layers corresponding to histology (T). Additionally, this technology allows identification and FNA sampling of locoregional lymph nodes (N). Distant metastases (M), as defined by the presence of positive celiac node involvement or liver metastasis, can also be adequately visualized with this technology.
T-Staging
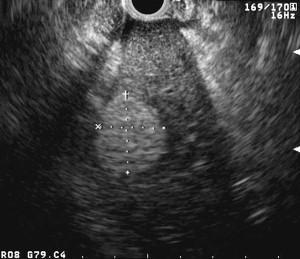
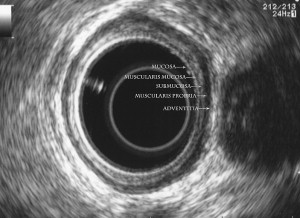
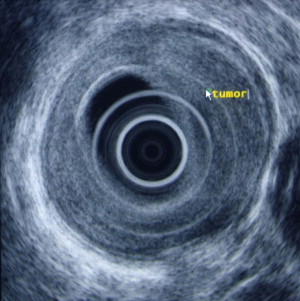
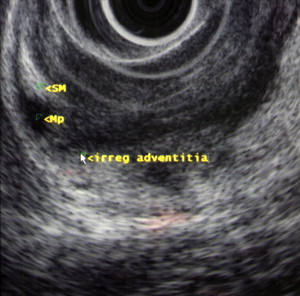
Identification of the normal ultrasonographic layers of the esophagus allows recognition of the degree of tumor infiltration ( Fig. 1 ). The first hyperechoic layer corresponds to the mucosa, the second hypoechoic layer corresponds to the muscularis mucosa, the third hyperechoic layer corresponds to the submucosa, the fourth hypoechoic layer corresponds to the muscularis propria, and the fifth hyperechoic layer corresponds to the adventitia. The clinical classification for the depth of tumor invasion (T) is assessed as follows: invasion to the second ultrasound layer (T1a), invasion up to third ultrasound layer (T1b), invasion limited to the fourth ultrasound layer (T2) ( Fig. 2 ), invasion beyond the fourth ultrasound layer (T3) ( Fig. 3 ), and invasion of adjacent structures such as the aorta, azygous vein, or trachea (T4).
N-Staging
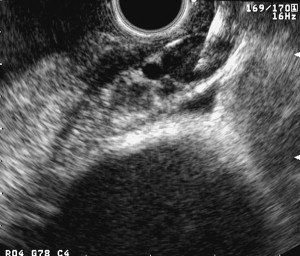
Lymph node involvement is designated by either N0 or N1, corresponding to whether the node is affected (N1) or not (N0). EUS allows visualization of left paratracheal, subcarinal, aortopulmonary, and paraesophageal lymph nodes. The accuracy for EUS-lymph node staging is reported to be in the range of 70% to 80%. Lymph node EUS features suggestive of malignancy include size greater than or equal to 1 cm, rounded shape, well-delineated borders, and a hypoechoic internal structure ( Fig. 4 ). If all of these features are present, malignant lymph node invasion can be predicted with an accuracy of 80% to 100%. However, all four of these features are only present in one-fourth of cases, thus making it difficult to predict lymph node metastasis in the remaining three-fourths of cases. An erroneous classification for N staging has been reported at 25%, mainly because of nonspecific peritumorous inflammatory appearances.
The development of EUS-FNA has increased accuracy of nodal staging. In a multi-center trial of 171 patients with upper gastrointestinal lesions, EUS-FNA for N staging had a sensitivity of 92%, specificity of 92%, positive predictive value of 100%, and a negative predictive value of 96%, with an overall accuracy of 92%. In another series, EUS-FNA compared with EUS had an accuracy of 87% and 74%, respectively. However, there are instances where the lymph node is inaccessible without traversing the primary tumor. FNA in these cases is not recommended secondary to the risk of sample contamination and false-positive results. The current standard of care is to perform EUS-FNA whenever feasible to maximize staging accuracy.
M-Staging
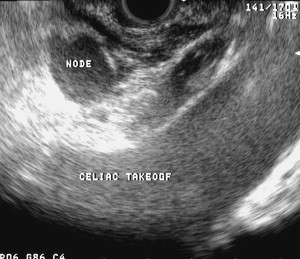
Perhaps, the most important role EUS plays in the staging of esophageal cancer is the identification of malignant celiac lymphadenopathy. Its presence denotes M1a or stage 4 disease and usually deems patients inoperable. Celiac lymph nodes are defined by their location within a 2-cm area from the celiac trunk ( Fig. 5 ). In a study by Puli and colleagues, where data was extracted from 25 studies, the pooled sensitivity of EUS in the detection of celiac adenopathy was 67.2%, while the specificity was 98.1%. Reed and colleagues evaluated the clinical impact of performing EUS after CT in a group of 62 patients. EUS detected abnormal celiac lymph nodes in 19 patients, with cytologic confirmation in 15. On the other hand, CT only revealed suspicious celiac lymph nodes in two patients. This enhanced detection of celiac adenopathy is crucial, as its presence portends a poor prognosis; potential unresectability, and consequently, palliation is offered. More recently, some institutions have treated M1a disease with induction chemoradiation followed by surgery with the goal of cure. However, using this aggressive approach, the vast majority of these patients died secondary to their disease. Positron emission tomography (PET) has also been compared with EUS in the detection of celiac adenopathy. Flamen and colleagues showed that PET significantly improved the detection of M1 disease over that achieved by combination spiral CT and EUS (82% versus 64%, respectively; P = .004), but did not improve the accuracy of locoregional lymph-node staging (33% versus. 81%; P = .027).
Lastly, comprehensive EUS staging of esophageal cancer includes inspection of the liver for metastatic disease ( Fig. 6 ). Studies have shown that EUS can pick up metastases, especially in the left lobe of the liver, which may not be detected by CT. For example, the study performed by McGrath and colleagues looked at 98 patients who underwent EUS staging for a new diagnosis of cardial or esophageal cancer. Of the 41 patients who underwent high-resolution helical CT scanning, EUS detected three (7%) malignant liver lesions not detected by CT. Therefore, it has been recommended that dedicated left-hepatic lobe examination should be performed in all patients undergoing upper gastrointenstinal EUS for the staging or diagnosis of malignancy. Other less frequent sites of metastases that can be detected by EUS include the left adrenal gland and malignant ascites ( Fig. 7 ).