Chagas disease
Tumors (e.g., leiomyoma, Hodgkin’s disease, gastric carcinoma; “pseudoachalasia”)
Sarcoidosis
Hereditary cerebellar ataxia
Eosinophilic esophagitis
Hirschsprung’s disease
Chronic idiopathic intestinal pseudo-obstruction
Multiple endocrine neoplasia type 2b
Miscellaneous (e.g., juvenile Sjogren’s syndrome, moyamoya disease, Ondine’s curse, autism)
Clinical Presentation
The clinical picture of achalasia depends on the duration of symptoms and the age of the child at the time of the diagnosis [69]. It has been reported that childhood achalasia is more common in males and is largely occurring in school-age children (7–12 years). However, achalasia may occur also in early infancy. Cases have been also reported in premature neonates as young as 29 weeks of gestation and as small as 900 g [70, 71]. The mean duration of symptoms prior to diagnosis was less than 3 years in 80 % of the children, and the chief complaints are represented by dysphagia and emesis [9] . Table 11.2 summarizes the clinical symptoms of the children reported in the literature [72]. Noteworthily, children younger than 5 years of age present more frequently with vomiting, whereas dysphagia is the predominant complaint in older children [69].
Table 11.2
Chief complaints in childhood achalasia
Chief complaints in childhood achalasia | Frequency (%) |
|---|---|
Regurgitation/vomiting | 80 |
Dysphagia | 76 |
Loss of body weight | 61 |
Respiratory tract symptoms | 44 |
Thoracic pain | 38 |
Faltering growth | 31 |
Regurgitation at nighttime | 21 |
Dysphagia is progressive and initially confined to solids, and in later stages to both solid and liquid. The child usually reports a sensation of food getting “stuck” in the esophagus (“chest”) which is usually relieved by multiple swallowing efforts or by washing the food bolus down with liquids. The troublesome deglutition leads to limited oral intake (food refusal) and subsequently in suboptimal weight gain (failure to thrive) or weight loss. As the disease progresses, the esophagus becomes dilated and the patient may experience regurgitation of undigested food or saliva that accumulates at nighttime while the child is asleep, leading also to episodes of recurrent coughing and chocking. The latter may predispose the child to a significant risk of chest infections or even sudden death due to aspiration of esophageal contents [73].
Diagnostic Approach
Diagnosis is often delayed due to several factors, including its low incidence, and the inability of younger children to report the symptoms, which are often nonspecific. Moreover, most of the related symptoms and signs, such as emesis, respiratory involvement, and failure to thrive are commonly attributed to gastroesophageal reflux (GER) disease. Therefore, a detailed history and a careful clinical examination are paramount as they can expedite the diagnostic process by guiding the physician in the correct choice of investigations which will eventually establish the diagnosis. Some individuals will have associated alacrima and adrenal insufficiency (AAA syndrome) and may have dermal hyperpigmentation secondary to high blood adrenocorticotropic hormone (ACTH) concentrations. Radiological, endoscopic, and manometric procedures represent the diagnostic arsenal and are the recommended modalities for the diagnosis of achalasia [4]. Esophageal achalasia needs to be distinguished from other conditions that present with regurgitation, vomiting, and dysphagia. Table 11.3 presents the entities that physicians should include in the differential diagnosis of esophageal achalasia [4, 63, 74–82].
Table 11.3
Differential diagnosis of esophageal achalasia
Gastroesophagal reflux disease |
Esophageal stricture |
Eosinophilic esophagitis |
Asthma |
Tumors (pseudoachalasia) |
Rumination syndrome |
Eating disorders |
Chagas disease |
Miscellaneous |
Radiology
Upper gastrointestinal (GI) contrast series (esophagography/esophagogram/barium swallow) provide a convenient way to assess the anatomy of the upper GI tract. They are usually readily available and as a result can expedite the diagnostic assessment or confirm the diagnosis in many of the suspected cases of achalasia. In spite of its undisputed usefulness, reports from adult studies showed that esophagogram can be nondiagnostic in up to one third of cases [83]. These data however were not substantiated in the pediatric population where 92 % of the studies demonstrated abnormal findings [9].
The esophagogram usually reveals various degree of esophageal body dysmotility with or without esophageal dilation, narrowing of the lumen at the level of esophagogastric junction (EGJ; “bird-beak” or “rat-tail” appearance), poor emptying of the contrast material into the stomach, and prominent tertiary contractions in the case of vigorous achalasia [9, 84–87]. (Figs. 11.1, 11.2, 11.3) Esophagography supports the diagnosis in the case of an equivocal manometry and can also demonstrate findings of end-stage achalasia (e.g., tortuosity and angulation of the esophageal body, megaesophagus) which may modify the clinical decision regarding the most appropriate therapeutic approach [4, 88–92]. Additionally, radiology can be used as an objective tool to evaluate the response to treatment (the so-called timed barium esophagogram (TBE), which measures the height of the barium column in the upright position after an ingestion of a large barium bolus) [93, 94], and as a predictor of treatment’s efficacy [95–97].
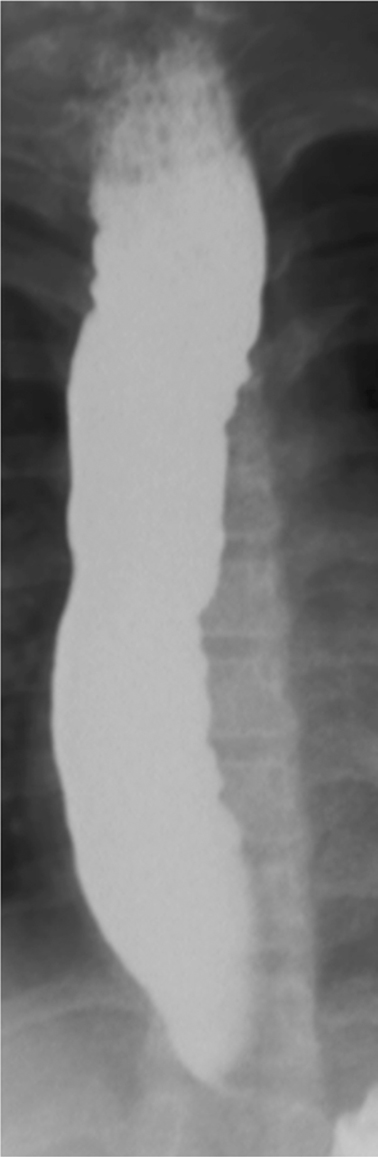
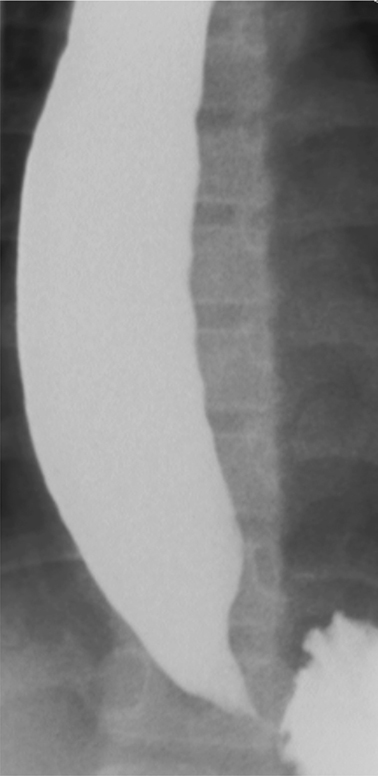
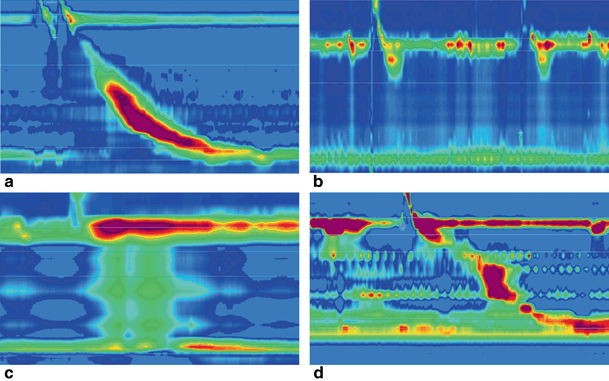

Fig. 11.1
Image of esophagus from barium esophagram in 11-year-old female with achalasia. There is food residue in the upper esophagus. The esophagus is dilated and shows numerous irregular contraction waves (tertiary contractions)

Fig. 11.2
Image of lower esophagus from barium esophagram in 11-year-old female with achalasia. The lower esophagus is dilated; there is tapered (“beak-like”) narrowing of the gastroesophageal junction

Fig. 11.3
High-resolution esophageal manometry in a healthy (a) and three achalasic children. Type I (b) is characterized by absence of distal pressurization to greater than 30 mmHg. In type II (c), pressurization to greater than 30 mmHg occurs in at least two of ten test swallows, whereas patients with type III (d) disease have spastic contractions with or without periods of compartmentalized pressurization
Upper GI Endoscopy
Endoscopy has an important role in the diagnosis of achalasia as it rules out clinical entities that can mimic achalasia, such as eosinophilic esophagitis and other causes of pseudoachalasia. It can also alter the initial working diagnosis for patients erroneously treated for GER [4, 98, 99]. The major endoscopic findings raising a high index of suspicion for achalasia include esophageal dilation, presence of retained saliva or food along with resistance while negotiating the LES. Mucosal abnormalities detected during endoscopic examination may be due to food stasis and candida infections. However, it is not unusual to witness an unremarkable upper GI endoscopy in the early stages of the disease [4].
Of significant note, an increased number of eosinophils has been reported in the esophageal biopsies of achalasia patients [100]. While the interplay among achalasia and esophageal eosinophilic infiltration needs to be further elucidated, it has been suggested that the latter does not represent a distinct clinical entity. Thus, the presence of esophageal eosinophils in patients complaining of dysphagia may warrant further diagnostic work-up to rule out other potential etiologies, including achalasia [101]. High-resolution manometry (HRM) is considered an invaluable tool for discriminating these two entities as they generally have distinctive motor patterns [102–104].
Manometry
Esophageal manometry provides a highly sensitive and specific method for defining the esophageal motility pattern [105]. This is especially true for the combined high-resolution esophageal impedance manometry [106]. Pediatric patients are assessed with a standardized protocol that involves single and multiple rapid wet swallows and solid swallows [104]. The esophageal motor abnormalities found in patients with achalasia are classified in the three subtypes of achalasia according to recently introduced Chicago classification [107]. All three subtypes are characterized by elevated integrated relaxation pressure (IRP—a variable which quantifies residual LES pressure): type I is defined by completely failed esophageal body peristalsis (Fig. 11.3b), in type II there is abnormal peristalsis accompanied by pan-esophageal pressurization in more than 20 % of the test swallows (Fig. 11.3c), and type III also demonstrates an impaired contractile pattern with fragmental preservation of distal peristalsis or presence of premature (spastic) contractions in more than 20 % of the swallows (Fig. 11.3d) [107]. The recently published results of the “European achalasia trial” demonstrated that HRM may facilitate clinical decision regarding the most appropriate initial treatment according to the manometric subtype [108]. The importance of HRM is highlighted by the fact that it can detect subtle changes in the esophageal motility dynamics and thereby aid in adding an organic element to conditions presenting with dysphagia and previously described as functional in nature [109]. Furthermore, some data suggest that intraoperative HRM along with the novel imaging tool “EndoFLIP” are safe and useful techniques in determining the adequacy of Heller’s myotomy , and therefore decreasing potential future recurrence of symptoms [110–113].
Lastly, dysfunction of the upper esophageal sphincter (UES), such as an increased resting pressure, shorter relaxation after swallowing, and shorter interval to pharyngeal contractions after UES relaxation, has been demonstrated in achalasia patients. The clinical significance of such findings is still not well understood [114, 115].
Management
The therapeutic management of achalasia includes pharmacological, interventional, and surgical options. None are curative but they aim in providing symptomatic relief of the EGJ obstruction without addressing the esophageal body dysmotility. However, there is some evidence suggesting potential improvement of the esophageal motility after surgical treatment for achalasia [116]. A simplified management approach is reported in Fig. 11.4.
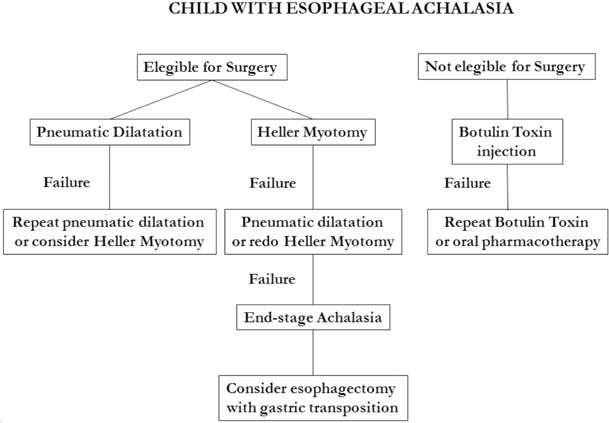

Fig. 11.4
Suggested therapeutic algorithm for children with achalasia
Pharmacological Therapy
Oral pharmacological treatment is the least effective in managing achalasia [117]. Isosorbide dinitrate and nifedipine act by relaxing the smooth muscles and by blocking the calcium channels, respectively; both reduce the LES pressure [118, 119]. They are administered prior to meals and are considered a temporary measure until a more definitive treatment, either dilatation or surgery, is provided. Oral pharmacotherapy alone is recommended in adult patients who are not willing or eligible for dilatation or surgery and in whom endoscopic botulinum toxin injection (EBTI) has previously failed [4].
Endoscopic Botulinum Toxin Injection
Intrasphincteric botulinum toxin injection blocks the presynaptic cholinergic terminals in the LES and therefore inhibits the secretion of acetylcholine at the neuromuscular junction. The latter results in chemical denervation with consequent opposition of the excitatory effect of acetylcholine and disruption of the relentless contraction of LES [4]. The experience of EBTI in the treatment of childhood achalasia is relatively limited. The method involves the endoscopic injection of 25 units of botulinum toxin in each one of the four LES quadrants (100 units in total) [69]. Similar to pharmacotherapy, EBTI offers a short-term symptom relief, as pneumatic dilatation (PD) or Heller myotomy (HM) will be eventually required for a more sustained clinical improvement [120, 121]
Pneumatic Dilatation
The goal of performing a forceful esophageal dilatation is to stretch and disrupt the LES fibers to such an extent that it alleviates EGJ obstruction without inducing GER [4, 122]. The procedure is performed under general anesthesia, and patients undergoing dilatation need to be eligible for surgery in the unfortunate event of esophageal perforation [4]. A balloon dilator is inserted with fluoroscopic or endoscopic guidance [67, 123]. The non-radiopaque polyethylene balloon dilators are preferred over the bougienage or the standard balloon dilators as the latter are not effective enough in rupturing the LES fibers and achieving adequate symptoms’ control. The modern dilators are of graded size (3.0, 3.5, 4.0 cm); however, for technical reasons PD is usually performed only in children older than 6 years of age or those weighing over 20 kg [4, 67]. After the correct position is confirmed, the balloon is distended for 60 s with pressures that vary among institutions from 2–12 psi [67, 123]. Balloon distention until obliteration of its waist while under fluoroscopy is more important for achieving symptom control than balloon distention time [4]. It is advisable that all patients are observed for a certain period of time prior to discharge home, and if symptomatic, they need to be assessed with a gastrografin esophagogram to exclude potential esophageal perforation. The incidence of esophageal perforation in adults who underwent PD for achalasia was reported between 4–12 % [123]. No cases of post-PD esophageal perforations were documented in recent pediatric studies [67, 123–125]. Small asymptomatic perforations are managed conservatively (intravenous antibiotics, total parenteral nutrition) whereas immediate surgical intervention is mandated for free perforations [126, 127]. The majority of perforations occur during the first dilatation most probably due to inaccurate placement and distention of the dilator; GER has been reported in 15–35 % of the patients who underwent dilatation for achalasia treatment [73, 128]. The latter is of important significance as recurrence of symptoms in post-PD patients may be due to reflux-related distal esophageal stricture. Thus, treatment with proton pump inhibitors (PPI) is warranted in post-PD patients complaining of GER symptoms. Other potential post-PD complications include aspiration pneumonia and pain that may require prolonged hospitalization [124].
PD achieves adequate control of symptoms ranging from 67 % after the first dilatation to 87.5 % after subsequent procedures [67, 123]. Available data suggest that although PD achieves immediate symptom relief, it bears a significant likelihood of symptoms’ recurrence that will require subsequent interventions in the form of either repeat PD or surgery [69, 124, 125, 129]. It is worth mentioning that minimally invasive surgical techniques have challenged the role of PD as the first-line treatment in childhood achalasia, and the recently published data question the efficacy of PD as HM was proven to be significantly superior to dilatation [69, 124, 125, 130].
Surgery
The mainstay of surgical treatment for achalasia is Heller’s myotomy , which was first described in 1913 [131]. Over the past decades, HM has evolved from the open thoracotomy to the minimally invasive laparoscopic technique, which has become the procedure of choice among surgeons due to its high efficacy, faster recovery, and minimum morbidity rate [124, 129, 130, 132]. The resolution of symptoms after HM largely depends on the length of the myotomy; this should be long enough to diminish the EGJ obstruction but not excessively long so that it induces postoperative GER. The optimal length of HM in children of different age groups has not been established yet. A recent report advocates an incision length between 4–6 cm that ends 5 mm distally to EGJ along with minimal mobilization of the adjacent anatomical structures [133]. The role of both “EndoFLIP” and intraoperative manometry appears to be very promising in determining the optimal myotomy length [110–113].
The addition of a fundoplication after HM and also the type of the anti-reflux procedure are still a matter of debate. An anti-reflux procedure reduces the risk of postoperative GER but at the same time increases the likelihood of dysphagia, as it creates a high pressure zone to an esophageal body with an already impaired motility [134–136]. An anti-reflux procedure may be warranted when a wide mobilization of the esophagus is inevitable in order to technically facilitate the myotomy [133]. Regarding the most appropriate type of the anti-reflux procedure there are data revealing that a loose Nissen, Toupet, Dor, or Thal fundoplication along with HM fundoplication are safe and result in good outcomes in the treatment of childhood achalasia [90, 125, 130, 137, 138]. The American College of Gastroenterology advocates PPI treatment in achalasia adult patients complaining of heartburn post-HM in spite of the presence of a fundoplication [4].
Patients who have developed “end-stage” achalasia and failed myotomy are candidates for esophagectomy with gastric transposition [4, 90, 124, 125]. However, esophagectomy bears greater morbidity and mortality compared to laparoscopic HM; dysphagia requiring PD can still recur up to 50 % of the adult patients that underwent esophagectomy for “end-stage” achalasia [139].
Emerging Treatments
POEM is an acronym for “peroral esophageal myotomy”: it is a novel technique developed in Japan [140, 141], whereby the endoscopist performs an LES myotomy by creating a submucosal tunnel using a forward-viewing endoscope. The overall success in adult studies has been more than 80 % at 12-month follow-up [141]. There are limited data regarding the applicability, safety, and efficacy of this method in childhood achalasia. Experience from a case report and a small case series reveals excellent outcomes regarding its safety and efficacy at 12-month follow-up assessment [142, 143].
Follow-Up and Surveillance
Currently, there is a lack of consensus regarding the optimal short- and long-term follow-up of children treated for esophageal achalasia . It is however advisable that pediatric patients receive regular follow-up assessment to ensure reduction of symptom severity (Eckardt score) [146]; evaluation of the esophageal emptying with a barium esophagogram may be also considered [4].
Patients treated for achalasia have a normal life expectancy [147]. Despite an increased risk of esophageal carcinoma in patients with achalasia [148, 149], there are limited data to support regular screening for cancer. Thus, the American College of Gastroenterology and the American Society for Gastrointestinal Endoscopy do not advocate routine endoscopic surveillance for achalasia patients [4, 150]. Nevertheless, radiological or endoscopic surveillance is recommended by numerous authorities for the assessment of adult patients treated for achalasia as they are in an increased risk for developing end-stage disease with megaesophagus. This approach with regular evaluation in 3-year intervals is particularly proposed if the disease has been diagnosed for more than 10 to 15 years [151]. Clearly, more data are required for the implementation of such recommendations in childhood achalasia.
Conclusion
Childhood esophageal achalasia is an enigmatic disease. Contemporary diagnostic modalities (upper GI contrast series, endoscopy, HRM) offer rapid and accurate diagnosis. Current data suggest that laparoscopic HM combined or not with an anti-reflux procedure seems to be the therapeutic procedure that offers the most durable and sustained long-term symptom relief.
References
1.
Willis T. Pharmaceutice rationalis sive diatriba do medicamentorum operationibus in humano corpore. London: Hagae Comitis; 1674.
2.
Hurst AF, Rowlands RP. Case of achalasia of the cardia relieved by operation. Proc R Soc Med. 1924;17:45–6.PubMedCentralPubMed
3.
Kun L, Herbella FA, Dubecz A. 1913: annus mirabilis of esophageal surgery. Thorac Cardiovasc Surg. 2013;61:460–3.PubMed
4.
Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238–49, quiz 50.PubMed
5.
Ghoshal UC, Daschakraborty SB, Singh R. Pathogenesis of achalasia cardia. World J Gastroenterol. 2012;18:3050–7.PubMedCentralPubMed
6.
Azizkhan RG, Tapper D, Eraklis A. Achalasia in childhood: a 20-year experience. J Pediatr Surg. 1980;15:452–6.PubMed
7.
Mayberry JF, Mayell MJ. Epidemiological study of achalasia in children. Gut. 1988;29:90–3.PubMedCentralPubMed
8.
Marlais M, Fishman JR, Fell JM, et al. UK incidence of achalasia: an 11-year national epidemiological study. Arch Dis Child. 2011;96:192–4.PubMed
9.
Myers NA, Jolley SG, Taylor R. Achalasia of the cardia in children: a worldwide survey. J Pediatr Surg. 1994;29:1375–9.PubMed
10.
Vaezi MF, Richter JE. Diagnosis and management of achalasia. American college of gastroenterology practice parameter committee. Am J Gastroenterol. 1999;94:3406–12.PubMed
11.
Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–74.PubMed
12.
Dayalan N, Chettur L, Ramakrishnan MS. Achalasia of the cardia in sibs. Arch Dis Child. 1972;47:115–8.PubMedCentralPubMed
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







