The hepatitis C virus (HCV) is a leading cause of liver-related morbidity and mortality in the United States and other parts of the world. The epidemiology of the disease is highly variable between and within countries, and strategies to deal with HCV identification and treatment must be tailored to the geographic location and the political and economic environment of the region. Although great strides have been made in improving HCV transmission risk in blood supply products, new challenges related to changing patterns of disease incidence continue to require fresh evaluation and new approaches to disease prevention.
Key points
- •
HCV remains a leading cause of liver-related morbidity and mortality across the globe.
- •
Incidence, prevalence, and transmission risk factors vary considerably within and between countries.
- •
New infection risk in developed nations is generally caused by injection drug use and unsafe sexual practices but may be caused by medical procedures or other local parenteral or iatrogenic routes in developing nations.
- •
Regional politics, economic situations, and immigration add to changing patterns of HCV epidemiology.
- •
Continued evaluation and disease prevention strategies are needed to keep virus transmission in check.
Introduction
Hepatitis C viral infection (HCV) represents a significant public health burden, chronically infecting between 2.7 and 4.1 million people in the United States civilian population and at least 150 million individuals worldwide. Most of those initially infected with HCV develop chronic infection, with clearance rates mediated by sex, transmission route, and other concomitant infections such as HIV. Chronic HCV can lead to progression to liver cirrhosis in 15% to 20% of those infected within 20 years, resulting in severe outcomes such as end-stage liver disease and hepatocellular carcinoma (HCC). At least 35% of individuals on the wait list for liver transplant in the United States are infected with HCV, and global HCV-associated mortality estimates are close to 500,000 deaths per year. Although the advent of oral direct-acting antiviral therapy (DAA) has generated considerable enthusiasm for vastly improved control of the virus, diagnosis and treatment of populations in resource-poor settings and marginalized populations have not improved in tandem, suggesting an urgent need for continued refinement of epidemiology, cost-utility models, and targeted diagnostic strategies.
Introduction
Hepatitis C viral infection (HCV) represents a significant public health burden, chronically infecting between 2.7 and 4.1 million people in the United States civilian population and at least 150 million individuals worldwide. Most of those initially infected with HCV develop chronic infection, with clearance rates mediated by sex, transmission route, and other concomitant infections such as HIV. Chronic HCV can lead to progression to liver cirrhosis in 15% to 20% of those infected within 20 years, resulting in severe outcomes such as end-stage liver disease and hepatocellular carcinoma (HCC). At least 35% of individuals on the wait list for liver transplant in the United States are infected with HCV, and global HCV-associated mortality estimates are close to 500,000 deaths per year. Although the advent of oral direct-acting antiviral therapy (DAA) has generated considerable enthusiasm for vastly improved control of the virus, diagnosis and treatment of populations in resource-poor settings and marginalized populations have not improved in tandem, suggesting an urgent need for continued refinement of epidemiology, cost-utility models, and targeted diagnostic strategies.
Hepatitis C virus epidemiology: the first 20 years (1975–1995)
Hepatitis C Virus Unmasked
Early evidence of a hepatitis virus other than hepatitis A or B was described in patients undergoing blood transfusion in the 1970s as assays for identification of hepatitis A and B became available. This non-A, non-B hepatitis was further described in patients who had not received blood products, such as families in hepatitis-endemic regions in Costa Rica and drug users in Milan. Extensive analyses of blood donors were performed to identify factors associated with disease transmission. Serum alanine aminotransferase levels and the presence of hepatitis B anti-core antibody were linked to non-A, non-B transmission and by 1986 all blood in the United States was screened for these markers, despite questions about the overall efficacy. It was estimated that this antigen and its parent virus were responsible for up to 89% of post-transfusion hepatitis and it was identified as the causative agent in chronic liver disease in people with hemophilia (PWH) and other clotting disorders. It was demonstrated to be transmissible even from patients without active disease. However, it was not until a decade later that Choo and colleagues isolated the genetic material of the virus, opening the door for vastly improved methods of viral identification and eventual assignment to the Flaviviridae family. The development and application of both an enzyme-linked immunoassay (ELISA) for detection of anti-HCV antibodies and the refinement of the polymerase chain reaction to quickly identify and sequence the genetic material of HCV during 1990s enabled accurate observational research and genotypic characterization of the virus ; thus, the field of HCV epidemiology was born during this decade.
Hepatitis C Virus Epidemic: the Early Days
Once rapid and accessible screening methods for HCV were available, a tsunami of epidemiologic research began to emerge, describing the high prevalence and blood-borne transmission patterns of HCV in various patient types and cohorts. An early study reported that more than 80% of patients with nonalcoholic liver disease who were previously diagnosed with cryptogenic hepatitis were actually positive for HCV antibodies. Similarly, nearly 75% of patients at the New England hemophilia center were found to have anti-HCV antibodies. By 1993, nearly 90% of hemophiliacs in the United States were infected with HCV from exposure to contaminated clotting factor concentrates received before 1987. These patients experienced recurrent exposure to concentrates made from plasma pools prepared from 20,000 or more blood donors at a time when just a single-unit risk of infection approached 5%. Moreover, HCV-infected patients with clotting disorders were also at greater risk for other parentally acquired infections, such as human immunodeficiency virus (HIV) and hepatitis B virus (HBV). HCV was detected in other settings as well, such as renal dialysis centers. Risk factors such as injection drug use (IDU) and unsafe sexual practices were also implicated in infection; seroprevalence of anti-HCV antibodies reached 100% in an Australian population with long-term use and were similarly high in drug users in Amsterdam. Sexual spread of the virus was also posited as rates were higher in sex workers without known injection drug use than controls, although some studies demonstrated low rates of HCV among HIV-infected sex workers in countries with low IDU, including Uganda and the Democratic Republic of Congo.
In the United States, a 7-year study of non-A, non-B hepatitis surveyed 4 sentinel counties with the recently developed immunoassay. This pivotal study revealed that although the incidence of HCV was stable over 7 years, primary modes of transmission changed from transfusion to use of injection drugs over this period. This early report solidified IDU as an important risk factor for HCV transmission in the United States, and sounded a warning for the health epidemic yet to arrive. The link between HCV and HCC was also confirmed, with the relative risk for HCC ranging from 4.8 to 7.3 if positive for anti-HCV antibodies. From 1985 to 1987, solvent/detergent treatment of donor blood was implemented for coagulation factors, effectively eliminating transmission to patients with clotting disorders. Recommendations for donor screening for HCV antibodies followed, which limited viral spread from contaminated blood transfusion products. From this point on, HCV was and continues to be primarily transmitted through other blood-borne routes including IDU, unsafe or traumatic sexual activity, nasal cocaine use resulting in mucosal damage, and practices such as unsanitary tattooing.
Hepatitis C virus epidemiology: 1995 to 2015
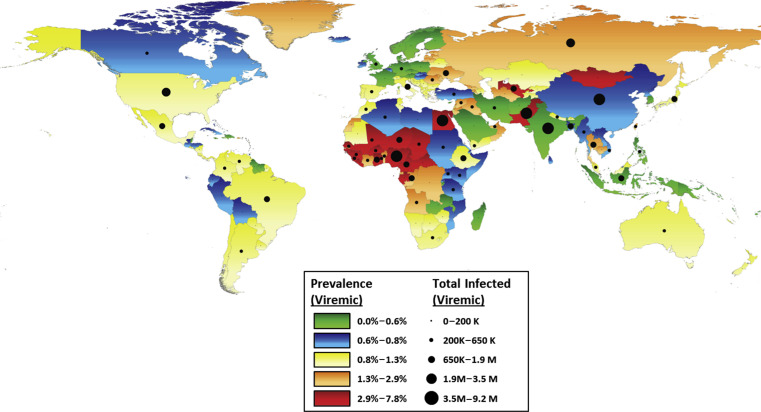
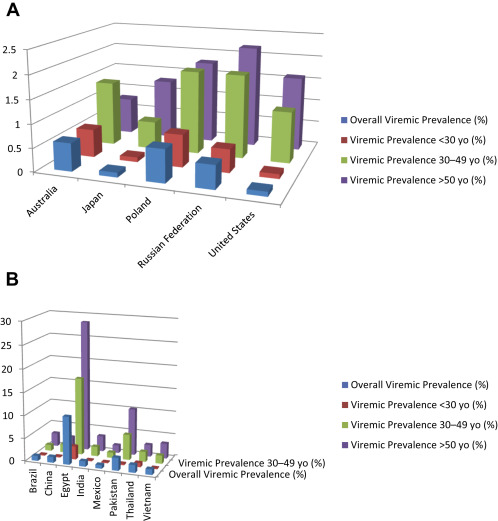
Current HCV distribution is highly variable, both intercountry and intracountry and region ( Fig. 1 ). In developed nations, such as North America, Western Europe, and Australia, the overall prevalence is less than 1.25%, according to a recent literature synthesis, but this review excluded studies focusing on special populations including people who inject drugs (PWID), PWH, HIV-positive, incarcerated, military veterans, and men who have sex with men (MSM). In developing nations, the population-based prevalence of anti-HCV may reach as high as 11% (Mongolia) to 15% (Egypt), although rates of viremia may be lower. Furthermore, poorer nations are less likely to be able to afford screening and DAA therapy. The public health threat posed by HCV is not limited to developing nations, however, as fluid immigration patterns may continue to facilitate the spread of HCV globally. A comparison of age-specific 2012 HCV prevalence for selected developed and developing nations is shown in Fig. 2 .
Current Hepatitis C Virus Epidemiology in Developed Nations
Generally, countries with a low prevalence of HCV (<2%) are developed nations where the population has access to sanitary conditions, less crowding, and adequate health care. However, variability in risk and transmission factors between these countries has led to differences in HCV epidemics, even among relatively similar countries.
For example, the most recent analysis of the National Health and Nutrition Examination Surveys (NHANES) for the years 2003 to 2010 in the United States estimated HCV prevalence of 1%, corresponding to approximately 2.7 million individuals. Previous NHANES analyses reported HCV prevalence of 1.6% between 1999 and 2003 and 1.8% between 1988 and 1994. These data and others suggest that peak incidence in the United States occurred during the 1970s and 1980s. Age-specific modeling has demonstrated that the greatest risk of infection for the US population is in those born between 1940 and 1965, and a recent analysis of US veterans revealed an HCV infection prevalence of 10.3% for those born between 1945 and 1965 versus 1.2% for those born after 1965.
A critical limitation of all of these studies, however, is that incarcerated and homeless individuals, who may be at high risk of infection from IDU or unsafe sexual practices, are excluded. Chak and colleagues conducted a comprehensive literature review to estimate the numbers of HCV-infected individuals from populations that were not accounted for in the NHANES surveys. They reported a revised estimate of between 5.2 and 7.1 million infected individuals in the United States. It is likely that age-specific prevalence would differ in some of these populations because of different risk factors than those in the 1945 to 1965 birth cohort, and that ongoing risk factors such as IDU and unsafe sexual practices may result in stable, rather than declining, incidence in these populations. Indeed, the current most frequent transmission route of HCV in the United States is IDU.
Japan has an overall prevalence of HCV infection comparable with the United States, but different transmission patterns have resulted in different age-specific prevalence. Most of the HCV burden in Japan was caused by contaminated blood products and unsafe medical procedures during the first half of the 1900s; molecular clock analyses have suggested that peak infection rates occurred as early as the 1920s. The highest age-specific prevalence in the general population is 2%, occurring in those more than 60 years of age. In contrast, prevalence in those less than 40 years of age is less than 0.5%. Risk factors in younger adults include IDU and tattooing, and this shift in transmission routes has led to alterations in genotype distribution in Japan. Although IDU is increasing in Japan, it is still relatively rare and is not expected to increase the overall burden of HCV or HCC. Low incidence and declining prevalence of HCV has resulted in a decrease in HCC in Japan between 1990 and 2003, from a peak of 41.5 cases per 100,000 persons in 1995 to 24 cases per 100,000 in 2003. Aggressive screening and treatment has largely contained the HCV epidemic in Japan.
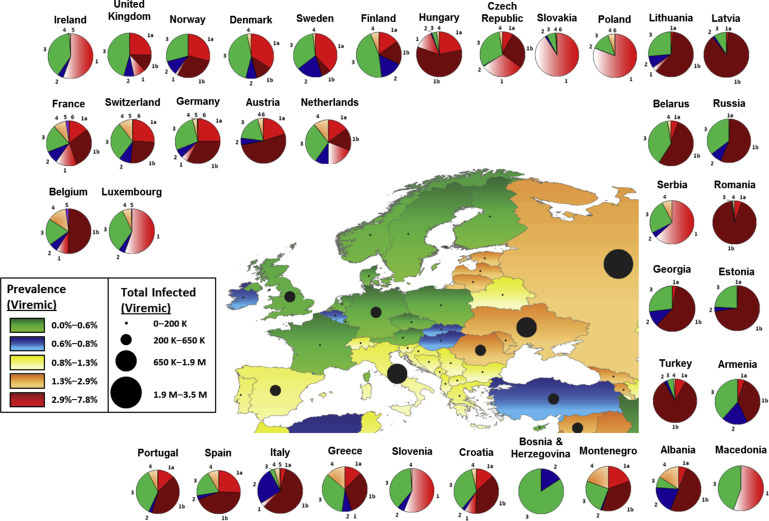
Accurate HCV incidence and prevalence rates and genotype distributions in Europe are difficult to attain because of large differences in diagnosis rates and reporting among European nations. With this caveat in mind, HCV prevalence in Western Europe as defined by HCV viremia ranges from 0.4% (Austria, Cyprus, Germany, Denmark, France, United Kingdom) to 1.5% (Israel, Italy), totaling 2.2 million individuals ( Fig. 3 ). Central and Eastern Europe have fewer available data on viremic individuals, but anti-HCV rates range from 0.7% (Czech Republic) to 4.5% (Moldova), yielding approximately 7.8 million anti-HCV-positive individuals. In general, IDU is the leading cause of transmission across Europe, although immigration from regions of higher prevalence, such as Egypt, have added to infection rates. A 2011 review of the literature regarding anti-HCV prevalence in PWID suggests that prevalence in that population is greater than 60% in nearly every European country; incidence rates up to 66/100 person-years have been reported. Cornberg and colleagues undertook a comprehensive systematic review of the literature on the epidemiology of HCV in Europe, Canada, and Israel. They describe decreasing HCV incidence in most countries in Western Europe. This decrease is partially the result of the changes in transmission routes, and partially a result of effective public awareness and health campaigns. For example, Denmark has a national centralized registry; some nations such as France have implemented national surveillance and prevention programs; and there is mandatory reporting of HCV infection in countries such as Sweden, Switzerland, and Poland. However, increasing incidence in many Eastern and Central European countries, including the Czech Republic, Poland, and particularly Russia, remains a serious concern, particularly with ease of travel and immigration throughout Europe. Romania in particular also merits attention. Epidemics of HIV, HBV, and HCV have been documented in Romanian children caused by the practice of whole-blood injections in orphanages in an attempt to improve nutritional status and re-use of needles and syringes. In 1993, 17% of children aged 0 to 3 years from Bucharest orphanages were HCV positive.
Australia also has implemented mandatory notification for HCV infection and has been reported to have HCV prevalence comparable with that of the United States (∼1%). Again, IDU is the primary mode of transmission. An elegant model of the Australian epidemic demonstrates a steady increase in incidence from 1960 to 2000, which was the peak of the epidemic (a decline after 2000 is attributed to a sharp decrease in heroin availability). Since 2004, prevalence has continued to decline among all adult age groups, but incidence is stable with the highest rates (between 6 and 8 notifications per year per 100,000 individuals) reported in those aged 20 to 29 years. Thus, the birth cohort experiencing the greatest HCV prevalence is younger than that in the United States, and the health burden from HCV can be expected to crest a decade later. Two-to three-fold increases in the numbers of HCV-positive individuals progressing through fibrosis to cirrhosis, HCC, and end-stage liver disease have been observed from 2003 to 2013. It is hoped that the advent of the DAA era for HCV will prevent further disease progression in patients who are successfully treated.
Current Hepatitis C Virus Epidemiology in Developing Nations
Given the challenges to gathering accurate epidemiology data in developed countries, it is not a surprise that the difficulties are even greater in developing nations. Many of these countries have little national health infrastructure for HCV screening or reporting, and limited resources for access to health care and treatment. Based on available data, prevalence varies greatly by country and within country. China, the largest by population size, has generally a low overall HCV prevalence, less than 1% in blood donors and the general population, as a result of stringent screening of blood product donors. However, pockets of high infection rates remain, particularly in older populations. Transmission seems to be primarily iatrogenic; dental work, cosmetic work, and blood transfusions have all been identified as risk factors. Family transmission, suggesting sexual transmission between spouses, has also been linked to infection risk. And as is the case with many countries, PWID and sex workers and clients remain at much higher risk for HCV infection. One study found an adjusted odds ratio of 60 for HCV positivity for female sex workers who had ever injected drugs, and prevalence in HIV-infected PWID is nearly universal. With a population size of 1.35 billion, even a conservative estimate of 0.5% HCV prevalence would yield 6.75 million infected individuals, and other reviews have estimated nearly double that number ; thus, HCV remains a primary health concern in China.
India is another developing nation with a population-based HCV prevalence rate of less than 2%, but a large population of nearly 1.3 billion individuals. Data from India are highly heterogeneous and generalizability is poor. Study populations tend to be either local or blood-bank donors, and donor screening data do not provide good estimates for an overall population as donors tend to be young and healthy. Currently there is no national surveillance or reporting system for HCV. The recent Consensus Statement of the HCV Task Force of the Indian National Association for Study of the Liver (INASL) has estimated overall prevalence of HCV between 0.5% and 1.5%, and has recommended the initiation of large-scale epidemiology studies to help fill this knowledge gap. Transmission in India has been primarily attributed to contaminated blood products, as mandatory blood product screening was not initiated until 2001. Unsafe injection practices have also been implicated. Puri and colleagues report that injections for common ailments are more frequent in India than in the developed world and inadequate sterilization is frequent; up to nearly 40% of cases of HCV in India has been attributed to unsafe medical injections.
Pakistan, although smaller than India and China, also has a sizable population and has higher estimated HCV prevalence of 4.7%. Hepatitis A infection is universal and hepatitis E is endemic, compounding the risk of liver disease. As in India, injections are popular remedies for illnesses and frequently used during medical procedures. Medical injection is the most commonly reported risk factor for HCV. A hepatitis sentinel surveillance system was implemented in 2011 to track all forms of viral hepatitis, with the aim of understanding the burden of liver disease caused by each. Local initiatives to improve awareness of HCV and the association with unsanitary injections may lower or stabilize the epidemic over the next several years.
Egypt has been reported to have the highest population-based prevalence of HCV, nearly 15% in individuals aged 15 to 59 years, and varies by region and age. Prevalence in Egypt has been shown to increase with lower education level, greater poverty, and with the number of household inhabitants. Nearly half of people aged 50 to 59 years are infected, and modeled incidence rates project more than 500,000 new cases in Egypt each year, stemming from a rate of ∼6.9 cases/1000 persons per year. Transmission in Egypt has primarily been attributed to a national anti-schistosomiasis campaign from the 1920s to the 1980s during which 3- to 4-month regimens of parenteral injections were given to children and young adults in parasite-infested areas. Although this practice was discontinued in the 1980s after the availability of oral anti-schistosomiasis therapies, iatrogenic transmission of HCV in Egypt remains a significant source of new infections. In 1 recent analysis of acute HCV in 4 Egyptian hospitals, hospital admissions were highly associated with risk of acute HCV. Vaginal or cesarean delivery, gum surgery, intravenous infusions, and injections all independently increased risk. However, this study was among nondrug users. In a recent meta-analysis of risk factors of HCV in Egypt, El-Ghitany and colleagues demonstrate that the highest odds of acquiring HCV are with IDU. Given the incidence and prevalence of HCV in Egypt and the health risks that infection poses, particularly superimposed on other infections such as schistosomiasis and endemic hepatitis viruses, prevention and treatment must be improved in this region.
Sub-Saharan Africa deserves discussion as it may contribute to as much as 20% of the global burden of HCV. High rates of seropositivity have been reported, up to greater than 8% in Nigeria, but wide variation in viremia has led to speculation of a high false-positive rate from immunoassays. However, a recent report has confirmed rates of viremia between 75% and 88% in a blood donor population in Ghana. It is possible that pockets of high transmission exist as a result of specific risk factors such as home births, tribal scarring, and traditional (versus hospital) circumcision.
Overall, HCV epidemiology in developing nations is highly heterogeneous. Different countries have idiosyncratic histories that mediate the risk of HCV transmission ( Table 1 ). Data are sparse and centralized screening, reporting, and treatments for HCV are largely absent. Diagnosis rates in countries such as India and the Slovak Republic are estimated to be as low as 5%, whereas in developed countries with registries such as Finland and Norway, most of their infected population has been diagnosed. Current unrest in many of these parts of the world is likely to contribute to factors that facilitate HCV spread: iatrogenic transmission from trauma; IDU; unsanitary conditions caused by poor water and electric supply; overcrowding; and lack of financial resources and infrastructure. India and Egypt combined are projected to result in nearly half a million cases of end-stage liver disease or HCC by 2030 ( Fig. 4 ). Public health measures are imperative to stem the flow of HCV both within and from these countries and resource allocation will be required to screen, diagnose, and treat patients early in the infection for prevention of a larger health burden over the next 2 decades.







