Increased number of mucosal eosinophils (subjective, not absolute quantitative threshold is defined except for eosinophilic esophagitis)
Degranulated eosinophils
Intraepithelial eosinophils (surface and gland/crypt epithelium)
Eosinophil surface layering
Eosinophil microabscesses
Epithelial degenerative/regenerative changes
Marked basal layer hyperplasia
Elongated papillae
Lamina propria eosinophilia and fibrosis
Foveolar/crypt hyperplasia
Villous atrophy in the small bowel
Minimal acute and chronic inflammation
Eosinophils in muscularis mucosa, submucosa, or both
In EGE, the stomach and small intestine are the most frequently affected segments of the GI tract [82]. The characteristics of the eosinophilic infiltration in each gut segment are given below in Table 27.2 [12, 24, 44, 68, 79, 80, 83–91].
Involved organ | Mean eosinophil density in normal conditions | Minimum eosinophil count required for diagnosis | Histopathologic features | Clinical manifestations |
|---|---|---|---|---|
< 5/hpf | 15/hpf in the epithelial layer | Elongated papillae and basal zone hyperplasia of the epithelial layer with eosinophilic infiltration of the lamina propria and muscularis mucosae. Eosinophilic microabscesses | Esophageal dysfunction, including dyspagia, food impaction, and GERD-related symptoms | |
2/hpf in lamina propriaa No intraepithelial eosinophilsa | > 20–30/hpf | Sheets of eosinophils, edema, eosinophilic degranulation, cryptitis | Dyspepsia, nausea/vomiting, epigastric pain, gastric outlet obstruction, ascitis | |
10/hpf in lamina propria Minimal intraepithelial eosinophils | > 20–30/hpf | Sheets of eosinophils, edema, eosinophilic degranulation, cryptitis Eosinophilic infiltration of lamina propria, muscle fibers, and serosal layer Hypertrophic muscle layer | Gastric outlet obstruction, abdominal pain, diarrhea, weight loss, malabsorption findings, perforation, ascitis | |
13/hpf in lamina propriaa Minimal intraepithelial eosinophilsa | > 20–30/hpf | Sheets of eosinophils, edema, eosinophilic degranulation, cryptitis Eosinophilic infiltration of lamina propria, muscle fibers, and serosal layer Hypertrophic muscle layer | Abdominal pain, small-bowel perforation, small-bowel obstruction, ascitis | |
8–30/hpfa | > 20–50/hpf (depending on location) | Eosinophil and lymphocyte infiltration of the lamina propria and the presence of intraepithelial eosinophils in the crypts | Diarrhea, bloody diarrhea, abdominal pain Constipation | |
Unknown | Unknown | Non available data on literature | Jaundice, cholestasis, epiastralgia, altered liver function tests Dilated bile ducts Pancreatic mass |
In the case of gastric mucosal samples, a recent study has evaluated the diagnostic criteria of “histological eosinophilic gastritis,” asserting that in the absence of other known causes of eosinophilia, histopathological findings such as sheets of eosinophils, frequent involvement of the muscularis mucosa or submucosa, and a density of ≥ 30 eosinophils/hpf in at least 5 hpfs are suitable diagnostic criteria for EGE [85].
The association of histological eosinophilic gastritis and the presence of Helicobacter pylori has been addressed in the literature with contradictory results. Thus, although a slow decrease in eosinophil count after H. pylori eradication was described in the early literature [92], more recent reports show no association between this infection and eosinophilic gastritis [85, 93]. Still, current guidelines state that a diagnosis of eosinophlic gastritis can be established only if mucosal eosinophilia persists several months after successful eradication of H. pylori [16]. Finally, superinfection by the protozoa Isospora belli, a common opportunistic parasite in immunodepressed patients, has been described as an exceptional association in EGE [94], but should be excluded after a definitive diagnosis has been established.
Knowledge about primary eosinophilic enteritis has been limited by the inaccessibility of the different small-bowel segments to mucosal sampling. Thus, in endoscopic investigations of the small intestine, biopsy specimens can usually be obtained from the duodenum, the first part of the jejunum, and the distal ileum. Descriptions of eosinophilic infiltration in other segments of the small intestine mainly come from the surgical literature [95–97]. In the overwhelming majority of patients, an increase in the number of mucosal eosinophils in the small bowel appears together with eosinophilic infiltration into other segments of the GI tract and represents an expression of EGE [37].
Eosinophilic colitis is characterized by increased numbers of mucosal and intraepithelial eosinophils in colonic biopsy samples. Additional histopathological features suggestive of eosinophilic colitis are provided in Table 27.1.
AEPC represents a distinct condition which is reported exclusively in infants and young children and is related to the ingestion of foreign proteins [4]. One relevant histopathological difference between AEPC and EGE with colonic involvement is that in the former, the overall mucosal architecture is usually well preserved and the eosinophilic infiltration is typically more localized in the rectum [2, 16, 21]. Diagnostic criteria include eosinophilic epithelial infiltration with more than 60 eosinophils/10 hpf in the lamina propria, and involvement of the muscularis mucosae. However, as patients respond well to treatment when AEPC is suspected, rectosigmoidoscopy with biopsies is usually unnecessary [98].
Besides eosinophilic infiltration, other recognized diagnostic criteria for EGE include mucosal edema, eosinophilic degranulation, glandulitis/cryptitis, eosinophilic crypt abscesses, and chronic architectural changes [79]. In contrast, epithelial infiltration may not be a constant feature [85]. In fact, in 10 % of all EGID cases, the mucosa exhibits no diagnostic changes, even when the inflammation is identified in subjacent submucosa, lamina propria, or muscular layers. This is probably due to sampling errors in a disease that can often be patchy [21].
Clinical Manifestations of EGE
From its initial description [6], EGE has been recognized as an extremely heterogeneous disease with respect to its clinical presentation. In 1970, Klein et al. proposed a classification of EGE into three different arbitrary patterns based on clinical manifestations and the depth of the eosinophilic infiltrate into the GI tract wall [99]. This classification has been used by many subsequent authors because, when combined with the topographical location of the eosinophilic inflammation, it helps to predict clinical symptoms. (Table 27.3)
Table 27.3
Summary of eosinophilic gastroenteritis symptoms and common findings, according with the classification proposed by Klein et al. [91] in 1970
Forms | Estimated frequency | Maximal depth of digestive tissue involvement | Main affected organs | Main symptoms | Common findings |
|---|---|---|---|---|---|
Mucosal | 45–80 % | Mucosa and submucosa | Stomach and duodenum | Abdominal pain, weight loss, diarrhea, nausea/vomiting, iron deficiency, malabsortion, protein-losing enteropathy | Mucosal hyperemia, ulcerations, aphthae, thickness of folders |
Muscular | 12–30 % | Muscle layer | Stomach and duodenum | Nausea/vomiting, gastric outlet or small-bowel obstruction | Strictures, rigidity, dysmotility, and obstruction |
Serosal | 12.5–39 % | Subserosal and serosal layers | Any segment of the GI tract | Ascitis and peritonitis | Eosinophilic ascitis, intense peripheral eosinophilia Small-bowel perforation |
a.
Mucosal form: This form is characterized by involvement of both the mucosa and submucosa, leading to malabsorption-related symptoms such as diarrhea, weight loss, iron-deficiency anemia, and loss of protein enteropathy. The mucosal form represents the most common presentation of EGE, making up 45 % of cases in several series. It has recently been suggested that the mucosal form of EGE has become predominant in the past few years, with a recent series reporting a prevalence of over 80 % [44].
b.
Muscular form: This form appears when the eosinophilic inflammation extends deeper into the muscle layers, leading to digestive wall thickening and typical obstructive symptoms. Often, this form of the disease is diagnosed after surgical resection of the affected segment [68, 100]. Muscular affectation can also manifest with decreased small intestine and colonic motility, leading to constipation [101]. The muscular form has been estimated to comprise 12–30 % of all EGE cases [43, 79].
c.
Serosal form (or eosinophilic ascitis): When the eosinophilic infiltration permeates all layers of the GI tract wall and extends to the serosal covering, this causes the appearance of eosinophilic ascitis, which is defined by an eosinophil cell count of at least 10 % [102], but reaching up to 80 % [103]. Eosinophilic ascitis is generally considered the rarest form of EGE, but has been recognized in 12.5–39 % of cases in some studies [43, 79].
Although it is a fairly accurate tool, Klein’s “classic” classification should be broadened to include two important EGE-related conditions that have been described repeatedly in literature.
d.
Intestinal perforation: Limitations in the biopsy sampling of most segments of the small intestine have probably led to diagnostic delays in the subset of patients with predominant small-bowel affectation, justifying its presentation as a distinctive complication requiring surgical repair. Perforation in the context of EGE has been observed in every segment of the GI tract, including the esophagus [17, 19], stomach [96], duodenum [104], jejunum [97, 105, ileum [95],and colon [106]. The transmural involvement of the disease in this case is different from that of eosinophilic ascitis, but it was not included in the Klein classification. The cytotoxic effector function exerted by eosinophil granule proteins has been proposed as the underlying cause of tissue damage in these patients.
e.
Biliopancreatic involvement of EGE: Extraintestinal involvement in EGE has been repeatedly described as a form of cholecytopancreatitis with bile duct dilation and obstructive jaundice [79, 89–91, 107, 108], together with symptoms derived from gut inflammation. Duodenal mass effect can also lead to acute obstructive pancreatitis [109].
While primary EGE has been described in patients of all ages, the majority of cases are diagnosed between the third and fifth decades of life [82]. In contrast to EoE, which is clearly predominant in males [78], no definitive data are available on gender predominance in EGE in general, although eosinophilic ascitis and the underlying transmural EGE form have been described predominantly in women, occasionally triggered during pregnancy and/or after delivery [34, 103, 110–112].
Several clinical aspects of EGE are intriguing, but the lack of large case series makes it difficult to propose definitive conclusions. Still, studies conducted to date indicate that peripheral blood eosinophilia is frequent among sufferers of EGE, being found in up to 90 % of patients [113]. This condition is more intense and frequent in patients with mucosal and serosal (with ascitis) EGE than in those affected only up to the muscle layers [43, 53, 79]. In addition, 80 % of cases have a personal background of allergies, 50–62 % of which are food allergies [3]. In contrast, only 27 % of adult patients reported a family history of allergy; interestingly, this was limited to patients with mucosal involvement [44]. Finally, 16 % of patients had or currently have a relative also suffering from EGE [3].
More than half of the patients also exhibited increased IgE serum levels [113]. While all these atopic manifestations seem to be more common in mucosal and serosal forms [79, 86], they are also present in a high proportion of patients with muscular forms of EGE [44].
With regard to the topographical distribution of EGE, the stomach and duodenum have been proposed as the most frequently involved digestive organs. However, these are also the digestive segments most commonly examined by means of endoscopy, which may lead to bias. Indeed, virtually any segment of the GI tract may be affected, with 50 % of patients presenting concomitant involvement of the rectum and/or colon [112] and 30–50 % of patients exhibiting simultaneous esophageal eosinophilic infiltration [4]. Thus, large bowel-derived symptoms including bloody diarrhea (which can mimic inflammatory bowel disease) and symptoms of esophageal dysfunction (e.g., dysphagia) may coexist together with stomach and small-bowel-derived symptoms. Interestingly, infiltration of the lamina propria by eosinophils and their presence in the crypts of rectal mucosal biopsies of young children with constipation due to cow’s milk intolerance has been described [86] as an alternative to EGE-associated diarrhea.
Diagnostic Imaging Techniques in Eosinophilic Gastroenteritis
Although EGE and eosinophilic colitis were both described decades ago, data on the typical radiological or endoscopic appearance of lesions in patients come mostly from more recent reports.
Most of the endoscopic findings described in EGE tend to be nonspecific, with mucosal hyperemia and thickened gastric folds [44] being the most common. Areas of rough or nodular appearance, erosions, aphthae, and ulcers have also been described in the disorder while in some cases, the endoscopic results have been normal [79]. Findings from capsule endoscopy include multiple erythematous lesions, loss of villi [113], incomplete strictures with ulcerated mucosa alternating with preserved areas [114], and a mimicking of mucosal diaphragms with complete retention of the capsule [115]. One patient with eosinophilic ascitis showed a bluish discoloration of the deep layers of the intestinal wall without mucosal changes; the authors hypothesized that in this case, eosinophilic infiltration had respected the mucosa [116].
Radiological findings in EGE are equally nonspecific in two thirds of patients [44, 117]; double contrast radiology findings are usually normal [44], but may occasionally show thickened folds, irregular or serrated edges in the walls of the small intestine, nodular contrast defects, or slow contrast progression indicative of GI hypomotility.
The findings in the colonoscopies of patients with eosinophilic colitis tend to be modest and nonspecific, sometimes showing patchy erythematous changes, loss of vascular pattern, and superficial ulceration. In other cases, the mucosa appears to be normal. A biopsy diagnosis is thus essential for demonstrating eosinophilic involvement of the colon [118].
Natural History of Eosinophilic Gastroenteritis
In spite of the approximately 500 EGE cases described in the literature to date, very little research has focused on elucidating the natural history of the disease . A recently published French study analyzed the clinical characteristics and evolution of 43 adult patients with EGE who were followed for a mean period of 13 years [79]. The authors described three different evolutionary patterns (Fig. 27.1). (a) 42 % of patients suffered a single outbreak of EGE lasting < 3 months, a pattern confirmed in a recent population-based study which also describes this self-limited course as being present in a majority of middle-aged adult patients suffering from eosinophilic colitis [119], (b) 37 % of patients exhibited a recurrent pattern of disease, with an average of 5.2 flare-ups at extremely variable intervals, and finally (c) 21 % of patients had a continuous disease course with persistent symptoms. No other studies have determined the global relapse rate after the first flare-up, although high eosinophil blood counts at the time of diagnosis have been associated with an increased risk of disease recurrence [79]. No tumoral or myeloproliferative transformation was observed in any patient during follow-up. In fact, an association between EGE and malignancy has only been described once in the literature in a case study of a 69-year-old Japanese man with multiple gastric cancer and EGE who responded well to a total gastrectomy and prednisolone treatment [120] .
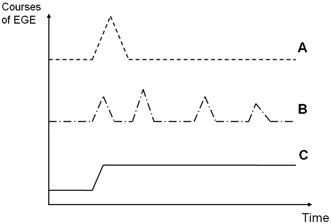

Fig. 27.1
Types of evolution of eosinophilic gastroenteritis (EGE). After a mean follow-up period of 13 years, Pineton de Chambrun et al. [79] identified three different types of evolution of EGE: a patients with a single outbreak of disease without recurrence (42 % of cases), b patients with a recurrent course characterized by multiple outbreaks and periods of complete remission lasting from 2 months to several years (37 % of cases), and c patients with a continuous course (21 % of cases). (Reprinted from Ref. [79], with permission from Elsevier)
Nevertheless, many questions regarding the natural history of EGE remain unanswered. For example, it has yet to be determined whether the disease in children and adults is the same or whether pediatric forms of EGE persist into adulthood. There is also a shortage of data concerning the ability of different therapeutic modalities to change the natural history of the disease, especially regarding the response to dietary-based therapeutic interventions [7].
With regard to AEPC in infants, the disorder is characterized by a benign course in which dietary elimination of the aggressor often resolves the symptoms within days [118].
Treatment of Eosinophilic Gastroenteritis
Heterogeneity in the clinical presentation, severity, and evolution of EGE, together with its low prevalence, has made it difficult to establish ideal treatment strategies for these patients. As in the case of other EGIDs, including EoE, no drugs have been approved specifically for the treatment of EGE and comparative studies of different therapeutic modalities are lacking. To make matters more confusing, both patient age (children or adults) and the medical specialty area in which they are attended tend to determine which treatments are administered. Basically, three major therapeutic modalities for managing these patients have been described in the literature; these include several dietary modifications to reduce the antigenic capacity of the diet, administration of drugs with anti-inflammatory and immunosuppressant effects, and finally, surgical intervention when complications arise.
Dietary Treatment
The most solid evidence of the efficacy of dietary treatment has been provided for infants (< 6 month) with AEPC. Since allergy to cow’s milk is identified as the major precipitant cause for this disorder, exclusion of cow’s milk from the diet of the lactating mother or from the infant’s diet is generally an effective therapeutic measure. Fortunately, clinical tolerance to cow’s milk develops in about 80 % of patients by 5 years of age [121].
The response to dietary therapies restricting certain foods has been recently studied in patients with EGE in a systematic review of literature [122], including 25 full-text articles and 5 abstracts. Overall, data from 86 individual patients (79 children) receiving 89 dietary interventions were retrieved. Data came from individual patients and short case series of up to 12 patients; most of documents were judged as having low methodological quality. Overall effectiveness in inducing clinical remission/clinical improvement was reported for 87.2 % of children and 88 % of adults included; however, no study objectively assessed changes in clinical complains by means of validated or nonvalidated instruments. Histological assessments after dietary treatment were made in only 20 individual children (22.5 %). Resolution or decrease in eosinophilic infiltration was documented in 16 cases (80 %), and changes were not noted in the 4 remaining patients. Authors concluded that, due to the relative lack of well-designed and high-quality studies, the unequivocal use of dietary treatment for patients with EGE and colitis cannot be supported, and they recommended undertaking further research .
Exclusive feeding with an amino-acid-based elemental diet , in which amino acids are used as the sole nitrogen source was used in 29 patients, all of them children [122]; clinical remission was reported in 75.8 % (22 patients). However, histological remission was assessed for only one patient with small-bowel involvement and in a further six patients with eosinophilic gastritis in a single research, 83 % of these having normalized mucosal biopsies [123, 124]. Semi-elemental diets (i.e., extensively hydrolyzed formulas with reduced antigenic capacities) as the exclusive nutritional source were used in only two patients [125]: Clinical and histological remission was documented in only one patient. A major advantage of elemental diets is their avoidance of steroids, thus preventing the potential adverse effect of growth retardation in these children. However, the many drawbacks of elemental diets, including their unpleasant taste, high nonadherence rates, high cost, and the psychological and social implications of complete avoidance of any kind of table food, restrict their use in clinical practice to infants and toddlers, among whom food restrictions are better tolerated, and only for the length of time required for food reintroduction with the goal of identifying specific dietary triggers. In any case, there is still no consensus in the literature as to which allergic evaluations or tests should be carried out on these patients .
Allergy testing–elimination diets are another therapeutic approach that consists of testing for food allergies with the aid of skin prick testing (SPT), atopy patch testing (APT), or determination of specific serum IgE against a given food in order to identify specific food triggers and eliminate them from the diet. This intervention strategy has rarely been documented in literature for EGE patients [126], since only three children and one adult underwent this treatment option: Clinical remission was described in every patient; biopsy samples after treatment were only available for one single child, who showed histological improvement [122].
A positive allergy test result did not predict whether a specific food was a disease trigger or not: A recent study carried out in patients with EoE, EGE, and healthy volunteers demonstrated that despite the general presence of a concomitant atopy in the first two groups, no differences in serum IgE levels for the most common food allergens were detected among the three groups [127]. According to consensus guidelines [78], allergy testing should be considered for patients with EGID to evaluate their allergic status. However, even though many individuals generate food-specific IgE antibodies that can be detected with allergy tests, they may still be clinically tolerant to various foods and have no allergic reaction following ingestion. This is probably because blood or skin tests are not specific for EGID; therefore, a wide variety of allergic diseases may be responsible for the results. In fact, food triggers can currently only be unequivocally identified by first documenting disease remission after specific food antigen avoidance, followed by disease recrudescence upon specific food reintroduction.
Most of the patients in the literature had been treated using different empiric approaches, which consisted of elimination of single foods (milk and wheat were the most common foods excluded) or combinations of foods considered to be of high risk of triggering an allergic response. A milk elimination diet, a treatment strategy used by 4 authors [128–131]on 16 individual pediatric patients, resulted in a symptomatic improvement rate of 62.5 %, with no histological assessment available. A gluten-free diet was used in two EGE patients with no clinical or histological benefits.
Several empiric elimination diets, which included empiric restriction of multiple foods, such as milk, cereals, egg, soy, seafood, and/or fruits, have been repeatedly used in the literature to treat EGE. The empirical elimination of the six most common food antigens from the diet (also called six-food elimination diet or 6-FED), and 7-FED (excluding red meats also) have been assessed in recent years. The aforementioned systematic review overall identified 34 patients with EGE or colitis have been given this dietary treatment and a symptomatic improvement has been reported in 29 (85.3 %) of these [122]. Histological assessments, however, have rarely been reported. A recent paper judged as of medium-/high-quality, reported histological remission of the disease after an empirical seven-FED in five out of six children with eosinophilic gastritis (83.3 %), and in two out of three patients after diet without one to three foods [123] .
Most of the reported cases retrieved in this systematic review presented a mucosal type of EGE, according to Klein’s classification [99]; muscular type was only described for one patient, serosal/transmural type was reported in a single case; in nine cases, the type of EGE was not reported. The low proportion of patients with muscular and serosal/transmural-type EGE prevented the authors to develop a comparative analysis. Regarding GI organ extension, no differences in clinical or histological remission/improvement were observed with regard to disease location or extension.
Once remission of EGE is achieved, specific foods should be reintroduced gradually, identifying problem foods by the reappearance of symptoms or through bioptic monitoring. Evidence of tolerance to offending foods after elimination and reintroduction has not yet been fully assessed. In the case of adult patients, allergic sensitization test results did not correlate with the specific foods responsible for the disease. Generally speaking, from the literature we can infer that the later EGE appears during childhood, the worse it responds to dietary modification [79] .
Drug Therapy
Corticosteroids have been by far the most widely used drugs for treating EGE in both children and adults [44, 80, 132]. These drugs also constitute the main treatment for patients in whom dietary therapy is not feasible or who fail to achieve improvement [2]. Prednisone, used at doses of 0.5–1 mg/kg/daily, has proven highly effective in the initial control of symptoms [44], eosinophilic tissue infiltration, blood hypereosinophilia, and also for controlling ascitis, as described in various studies and case reports. Clearly, the use of steroids in EGE does significantly more than merely control symptoms [113].Usually, after an initial treatment period of 7–10 days, the dose is gradually reduced until the drug is withdrawn after a period of up to 4 months.
Various series have described steroid-dependent patients in whom symptoms reappear during steroid tapering [113, 133]. These patients must resume taking previous doses, maintain remission by using low doses, substitute prednisone for budesonide [125, 126], or maintain remission with other antiallergic or immunosuppressant drugs [134]. Around 20 % of patients require maintenance therapy over time [125]. Budesonide has a better safety profile than prednisone and is especially useful in EGE affecting the distal small bowel and right colon [135], although it is also helpful in managing more proximal disease [126].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







