Fig. 46.1
Possible routes for EN
EN is used to preserve nutritional status, support normal growth, and treat malnutrition when oral feeding is inadequate or not possible. EN is more physiologic, usually safer, easier to administer, and less costly compared to parenteral nutrition (PN). Therefore, EN should be preferred to PN in infants and children with malnutrition and/or nutritional risk, when the intestinal tract is usable to provide sufficient nutrients for achieving optimal growth or catch-up growth [4]. EN may be administered rapidly as a bolus into the stomach, or more slowly over several hours as a continuous infusion into the stomach, duodenum, or jejunum. The underlying disease and patient tolerance are what determines whether to use bolus or continuous feeds. The physiological basis of continuous EN makes it of great interest in pediatric patients with feeding intolerance and other gastrointestinal (GI) disorders [4]. EN may be used as the sole source of nutrition or to just supplement a patient’s oral and/or PN intake. Also, depending on the indication, EN may be used daily or just on a periodic basis. While EN is normally initiated in the hospital, continuing it at home has become a common option. Home EN may be used long term or just as a temporary bridge until children achieve oral food intakes that support adequate growth and nutritional status. Even though home EN is an effective method of meeting a child’s growth and nutritional requirements, health practitioners should not disregard the mixed acceptance by families and sometimes negative impact on quality of life [5–7].
Physiological Basis of Continuous Enteral Feeding
Gastrointestinal Motility
The rate of gastric emptying, and secretion of pancreatic biliary fluids, is regulated by the infusion rate, calorie density, and osmolality of the enteral feeds [8]. In case of gastric administration of continuous feeds, a rate of continuous gastric emptying related to the infusion rate can be achieved if the infusion rate, calorie density, and osmolality of the mixture are not excessive. Steady-state gastric emptying of 1 kcal/mL formula can be maintained at infusion rates of ≤ 3 mL/min. When the infusion rate is excessive and higher than the gastric emptying rate, the risk of vomiting increases. As caloric load and/or osmolality of the formula increase, the gastric emptying rate is reduced to maintain a constant calorie load delivered into the duodenum. Thus, EN consisting of very calorie-dense formulas should initially be administered very cautiously. The individual nutrient composition of the formula has a lesser effect on gastric emptying function than the caloric density, except for the type of triglyceride: In fact, long-chain triglycerides (LCTs) cause greater delays in gastric emptying than medium-chain triglycerides (MCTs)
Gastric emptying is dependent on duodenal function. The effects of continuous enteral nutrition (CEN) on intestinal motility can be analyzed by manometry. Motor migrating complexes are observed in adults during CEN, as during fasting state [9]. In small preterm infants, duodenal motor activity is higher following slower infusion of gastric feeds than with rapid boluses, and this is associated with lower postprandial gastric contents [10]. During administration of jejunal feeds, energy loads at rates within the physiologic range of gastric emptying (≤ 4 kcal/min) initiate normal-motor small-bowel motor responses; however, increasing the osmolality (> 600 mosmol) has a significant inhibitory effect on small bowel contractile and propagative activity [11] and thus greater likelihood for intolerance. Very few data are available about the changes of colonic motility induced by CEN; the continuous gastric infusion of nutritive formula modifies the gastrocolic reflex. Gallbladder motility is maintained during CEN as assessed by increased serum cholecystokinin (CCK) and ultrasonography [11, 12]. Emulsified LCTs delivered to the duodenum have a potent stimulating effect on CCK release and gall bladder contraction [13]. Conversely, whereas dietary MCTs are more efficiently absorbed and rapidly metabolized compared to LCT’s [14], they are very weak stimulants for CCK release, gall bladder contractility, and hence luminal postprandial bile acid concentrations. Biliary complications such as sludge or cholelithiasis are rare during long-term CEN.
Digestive Secretion and Hormonal Response
Gastric secretion depends mostly on protein intake, and, in case of elemental diet , on amino-acids composition; secretory response is not influenced by carbohydrates but reduced by lipids. It has not been demonstrated whether or not the type of diet (i.e., elemental, semi-elemental, or polymeric) modifies gastric acid secretion [15]. Secretion of CCK and pancreatic polypeptide (PP) are maintained during CEN [12]. All forms of oral and enteral feeding stimulate pancreatic synthesis and secretion through CCK and PP. Pancreatic secretions can be reduced by 50 % if a low-fat elemental formula is used for duodenal feeding. Stimulation of pancreatic trypsin synthesis or secretion can be inhibited by delivering EN into the mid-distal jejunum [16]. The mechanism involves increased secretion of the ‘ileal-brake’ hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) [17, 18] which inhibit production of pancreatic secretions and motility in the proximal GI tract. Gastrin secretion is also maintained during CEN, but its response to protein load is decreased. Gastric or duodenal CEN stimulate insulin secretion depending on the type of infused nutrients. The glycemic and insulinemic response induced by EN parallels that of oral feeds and significantly lower than PN [16]; therefore, less risk for steatosis.
Effects of CEN on Mucosal Trophicity
The effects of elemental formulas (totally absorbed in the upper GI tract and providing minimal residue to the distal bowel) on small bowel mucosal trophism remain controversial. In studies, comparing intestinal trophicity and function in animals fed elemental diets versus regular chow, there was similar digestive function but significantly decreased mucosal mass in the distal bowel of animals-fed elemental diets. These changes could be the consequence of the almost complete absorption of nutrients within the proximal part of the small bowel, leading to lack of stimulation of the distal segment. This suggests an ability of EN to achieve bowel rest in the distal part of the bowel, providing efficient treatment for ileocolic inflammatory diseases.
Effects of CEN on Energy Expenditure and Feeding Tolerance
Thermogenic effect of feeding is related to the increase of energy expenditure for synthesis and secretion of digestive enzymes following ingestion of food. The increase in energy expenditure induced by CEN in normal subjects is lower than when the same nutrients are administered by bolus feed [19]. Finally, the slow and continuous administration of nutrients into the GI tract through CEN allows the achievement of optimal utilization despite intestinal illness. In fact, by changing the conditions of flow and of contact between the nutritive formula and the digestive tract, CEN may increase the capacity for intraluminal digestion and intestinal absorption. This feeding technique does not appear to provide benefit in patients without intestinal disease [20, 21]; however, it seems logical and efficient when the absorptive surface is reduced, for example, short bowel syndrome (SBS), villous atrophy, enterocutaneous fistula, or proximal enterostomy. CEN has been associated with better feeding tolerance , nutrient absorption, and growth than boluses or oral feeds in infants and adult patients with intestinal disease [22, 23].
Indications
Indications for EN are different from indications for PN, since the use of EN as nutritional support is based on normal or at least partially preserved gut functions. The decision tree for the route of feeding is determined based on aspiration risk, motility function of the stomach, and anticipated duration of need for EN support. See Fig. 46.2. EN can be used on any patient with normal GI absorptive function but unable to adequately be fed by mouth. The conditions commonly encountered are listed in Table 46.1.
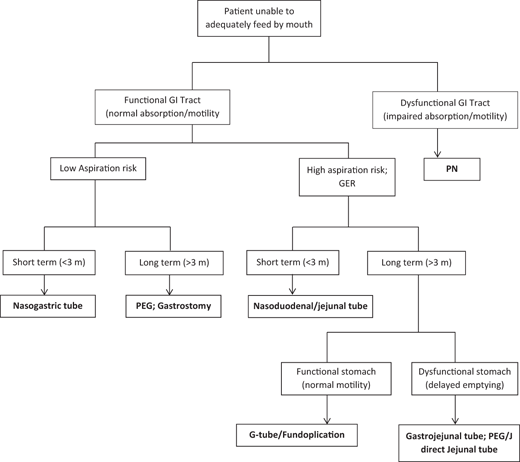

Fig. 46.2
Decision tree for the route of EN. GER gastroesophageal reflux, PN parenteral nutrition, GI gastrointestinal, PEG percutaneous endoscopic gastrostomy, PEG/J percutaneous endoscopic gastrostomy/jejunostomy, G-tube gastrostomy tube
Table 46.1
Conditions commonly managed with EN
Conditions with normal intestinal absorptive function |
Preterm infants |
Critically ill patients |
Nonorganic FTT |
Anorexia |
Inborn errors of metabolism |
Glycogen storage diseases, urea cycle defects |
Chylothorax |
Hypermetabolic states |
Head injury, graft versus host disease (GVHD), renal failure, congenital heart disease |
Digestive disorders |
Protracted diarrhea of infancy/childhood |
Short bowel syndrome |
Intestinal pseudo-obstruction |
Crohn’s disease |
Malabsorption disorders |
Cystic fibrosis |
Chronic liver disease |
Conditions with Normal Intestinal Absorptive Function
EN is required in cases of inability to eat normally, that is, in those situations that are secondary to structural or functional abnormalities of the upper GI tract or neurological impairment of the processes involved in sucking and/or swallowing (see also Chap. 20 on “Disorders of Sucking and Swallowing”). Esophageal diseases including esophageal atresia, fistula, or stenosis, often resulting from sequel of epidermolysis bullosa, are among the conditions that can benefit from EN, usually through gastrostomy or duodenal tubes [24, 25]. The choice of feeding through gastrostomy versus transpyloric tube must be assessed according to the patient age, disease, and condition (Fig. 46.2). Children with chronic diseases inducing immaturity or inability to feed orally, especially with sucking and swallowing troubles as seen in neurologically impaired children, with neuromuscular chronic diseases or cerebral palsy also require EN, using GTs.
Premature Infants
EN via nasogastric or orogastric tube is routinely used in premature infants younger than 32 weeks’ gestation because of uncoordinated suck, swallow, and breathing related to immaturity [26]. Human milk is the preferred feed because of its immunological benefits. Preterm infant formulas come fortified with protein, calcium/phosphorous, and a calorie density of 22–24 kcal/oz. to meet the high nutritional requirements of infants. A Cochrane systematic review of treatment trials did not provide evidence of any beneficial effect from transpyloric feeding over gastric feeding on feeding tolerance , growth, and development in preterm infants [27]. Therefore, gastric feedings administered by bolus or continuously is the preferred approach for EN in preterm infants, where its use appears to also have a role in preventing necrotizing enterocolitis [28] (see also Chap. 6 on “Enteral Nutrition in Preterm Neonates” and Chap. 7 on “Parenteral Nutrition in Premature Infants”).
Eating Disorders: Anorexia
Anorexia nervosa (AN) is a life-threatening psychiatric condition characterized by disordered eating behaviors, significantly lower than expected body weight, intense fear of becoming overweight, and a distorted body image. It is managed by a multidisciplinary team of health providers including psychiatrists, adolescent medicine pediatricians, nutritionists, and social workers. Indications for inpatient therapy include presence of suicidal or aggressive behaviors; severe bradycardia, hypotension, electrolyte imbalance, dehydration, and hypothermia; and medical complications, for example, seizures and pancreatitis [29]. Weight restoration is one of the major predicators for favorable short- and long-term outcomes in patients with AN [30]. Also, restoration of body weight is associated with improvements in malnutrition-induced cognitive impairments thus facilitating psychological and psychiatric therapy [31].
Nutritional support in AN remains very controversial. The oral feeding route is preferred; however, patients are given the option of voluntary or forced EN if resistant to therapy and/or severe malnutrition, that is, body mass index (BMI) < 13 kg/m2 in adolescents [32] and weight-for-height z score < − 3 in younger children [33]. Other options like GT and PN are considered in young children or severe and chronic cases [29]. Follow-up of adults treated with EN during adolescence did not show any long-term benefits or adverse outcomes on growth, recovery, or persistence of AN or risk for development of psychiatric comorbidities [34]. The initial range of prescribed energy intake varies from 10–60 kcal/day to 1000 kcal/day and > 1900 kcal/day with progressive increase during the course of hospitalization [29, 30]. The main complication of nutritional management is risk for developing re-feeding syndrome: hypophosphatemia, hypokalemia, edema, and increased hepatic transaminases. The risk factors associated with re-feeding syndrome include greater severity of malnutrition, abnormal electrolytes prior to re-feeding, use of EN or PN, and weight loss > 15 % within the preceding 3 months [29, 30]. Patients may be preemptively treated with phosphate supplements during the early phases of nutritional management.
Inborn Errors of Metabolism
EN is part of the standard therapy used to prevent biochemical abnormalities, metabolic decompensation, and catabolism in patients with inherited disorders of metabolism, for example, hepatic glycogen storage disease (GSD) and enzyme deficiencies of the urea cycle. Patients with GSD type 1 (glucose-6-phosphatase deficiency) develop hypoglycemia and compensatory biochemical abnormalities of lactic acidosis, hyperuricemia, hyperlipidemia, and platelet dysfunction all stemming from the primary defect of inability to dephosphorylate glucose-6-phposphate to free glucose. Managing GSD type 1 involves overnight continuous high-carbohydrate feedings and frequent daytime feedings supplemented with uncooked cornstarch [35, 36]. EN consisting of a glucose/glucose polymer solution or a sucrose-free, lactose-free/low formula enriched with maltodextrin may be used. EN should be started within 1 h after the last meal. Likewise, an oral or EN should be given within 15 min after discontinuation of the continuous EN because of the risk for hypoglycemia. Gastrostomy is contraindicated in patients with GSD type 1b because of complications in case development of inflammatory bowel disease or local infections [36]. Continuous EN should provide a glucose infusion rate of 7–9 mg/kg/min in children younger than 6 years, 5–6 mg/kg/min in children aged 6–12 years, and 5 mg/kg/min in adolescents [36]. Intermittent feedings of uncooked cornstarch may be used if continuous nighttime EN is not an option. No significant differences in biochemical parameters or growth have been found between patients with GSD type 1 receiving overnight continuous EN compared to scheduled feeds of uncooked cornstarch [37, 38]. The starting dose for uncooked cornstarch is 0.25 g/kg and optimal dose is 1.75–2.5 g/kg of ideal body weight every 6 h [36, 37, 39]. Patients with GSD type 3 (debrancher enzyme deficiency) have impeded glycogenolysis; however, gluconeogenesis is endogenously enhanced to maintain adequate glucose production. Therefore, nutritional management of patients with GSD type 3 involves frequent high-protein feedings during the day and a high-protein snack at night. Patients with GSD IV (brancher enzyme deficiency), GSD VI (phosphorylase deficiency), and GSD IX (phosphorylase kinase deficiency) respond to the to the high-protein diets similar to what is recommended for patients with GSD type 3 [40].
Inherited defects in urea synthesis are inborn errors of nitrogen detoxification and arginine synthesis due to defects in the urea cycle enzymes namely carbamylphosphate synthetase 1, ornithine transcarbamylase, arginosuccinate synthetase, arginosuccinate lyase, and arginase [41]. They may present at any age with symptoms ranging from poor feeding to coma shock and death. Other clinical symptoms may include lethargy, vomiting, ataxia, confusion, behavior changes, hypotonia or spasticity, hyperventilation (leading to respiratory alkalosis), and seizures. The main biochemical abnormalities are hyperammonemia and increased plasma concentrations of glutamine [42]. The symptoms of metabolic crisis are usually precipitated by protein intake in excess of what the patient can metabolize, catabolism of lean body mass resulting from intercurrent infection, trauma, inadequate energy intake, or inadequate intake of protein or essential amino acids . Management of patients with urea cycle defects involves a combination of carefully restricting protein intake and therapy with nitrogen-scavenging drugs, for example, sodium benzoate, sodium phenyl acetate, or sodium phenyl butyrate [41]. The nutritional management includes: (1) administration of sufficient energy to support anabolism, (2) restriction of protein intake to that tolerated by the patient without producing excess NH3, (3) provision of essential amino acids in adequate amounts to support growth, (4) supplementation of “conditionally” essential arginine or citrulline in all except arginase deficiency, and (5) provision of all required minerals and vitamins in adequate amounts for age [43]. During hyperammonemic metabolic crisis, the nutritional management consists of providing high-energy low-protein intakes [44]. Successful long-term management requires a dedicated metabolics dietician and physician to make dietary adjustments and closely monitor progress. Patients with neurological handicaps or developmental delays, feeding difficulties, poor appetite/refusal of food, compliance problems with the diet, and/or medications will require use of nasogastric or gastrostomy feeding tubes to ensure adequate intake [41, 45]. Patients in metabolic crisis with symptomatic hyperammonemia (> 500 μM/L) and/or lack of response despite 4 h of medical treatment should have management escalated to dialysis [41].
Hyper Metabolic States
Hypermetabolic states include patients with burns, cancer, and head injury. Much of the morbidity and mortality in severely burned patients is connected to the prolonged hypermetabolism and catabolism, impaired wound healing, and sepsis. Whenever, GI function permits, EN is superior to PN in patients with burns [46]. EN results in better regulation of the postburn catabolic hormones and inflammatory cytokine responses than PN [46, 47]. Furthermore, early EN support of patients with severe burns helps maintain gut mucosal integrity, which has the beneficial effect on reducing risk for enterogenic infections [48].
Graft Versus Host Disease
Traditionally, PN is given as the first option for nutrition support in children undergoing chemotherapy and/or bone marrow/stem cell transplant. The reasons cited range from intestinal injury and poor GI tolerance secondary to conditioning and myeloablative therapy; intestinal graft versus host disease (GVHD), oral mucositis, epistaxis, and parental refusal of EN. However, whenever GI function permits, EN is equally as effective as PN, associated with lower risk for infection, and more cost-effective [49–52]. A prospective study comparing EN and PN in children with bone marrow transplants had poor enrolment into the EN group. Initiation of EN prior to transplant was associated with better overall tolerance. The EN group was also less likely to develop cholestasis [51]. A more recent Cochrane database review of nutrition support in patients of all ages with bone marrow or stem cell transplants failed to find evaluable data that properly compared efficacy and superiority of EN versus PN. However, the overall findings suggested that in patients without GI symptoms, intravenous fluids and oral diet should be considered as preference to PN [53].
Renal Failure
Supplemental nutrition should be given to children with renal failure to promote positive nitrogen balance and meet energy needs [4]. Children with chronic renal failure are at risk for malnutrition and growth retardation from persistent anorexia, inadequate protein calorie intake, chronic metabolic acidosis, azotemia, hormonal and metabolic disturbances, and catabolic diseases associated with uremia, for example, infections. Long-term EN is effective in preventing growth retardation in children with chronic renal disease and persistent anorexia, especially if started before the age of 2 years [54, 55] but singly may not lead to catch-up growth [56]. There is a positive correlation between efficacy of dialysis and linear of children with chronic renal failure [57]. Therefore, the combination of aggressive nutrition support with whey protein-based formulas in children age < 2 years, whole protein enteral formula (1–1.5 kcal/mL) in older children [58], and enhanced dialysis is necessary for inducing growth in children with chronic renal failure [59]. Growth hormone therapy is recommended if there is persistent growth retardation despite adequate nutrition support [58, 60]. Ultimately, catch-up growth in height is mainly seen in children who undergo renal transplant before the age of 6 years [61].
Congenital Heart Disease
Inadequate calorie intake is the predominant cause of growth failure in infants and children with congenital heart disease. Different approaches for nutrition intervention are utilized during the preoperative, postoperative, and post-discharge periods, respectively [62]. Infants with cyanotic congenital heart disease and complex ventricular lesions are particularly susceptible to malnutrition and growth failure [63]. Approximately, 50 % of infants with surgically treated uni-ventricular lesions get discharged home on EN via nasogastric or GT [64]. The causes of malnutrition include an imbalance between energy intake and increased expenditure particularly in children with congestive heart failure. Infants with severe congenital heart disease have normal resting energy expenditure [65, 66] but increased total energy expenditure [67], thus indicating that they expend large amounts of energy above basal requirements, which places them at risk for inadequate calorie intake when feeding at normal rates. Perioperative injury to the recurrent laryngeal nerve may lead to feeding difficulties from swallowing dysfunction and vocal cord paralysis [68]. Other contributing factors include pulmonary hypertension, tachypnea and fatigue interfering with oral feeding, medications associated with anorexia, for example, diuretics, prescription of fat-restricted diets in patients who develop chylous effusions, and GI nutrient loss in patients who develop post-Fontan protein-losing enteropathy. Post-Fontan protein-losing enteropathy is managed with therapies directed at improving cardiac hemodynamics and controlling inflammation [69].
Digestive Indications
Since EN has a trophic effect on the intestinal mucosa, and helps maintain mucosal integrity, it plays an important role in the treatment of many digestive diseases, either replacing or complementing oral feeding. Digestive diseases leading to an anatomical or functional reduction of the absorption capacity of the small bowel represent the first group; they include SBS, protracted diarrhea with villous atrophy, and inflammatory bowel disease.
Severe Protracted Diarrhea of Infancy
A syndrome of intractable diarrhea of infancy was first described by Avery et al in 1968. Its definition, presentation, and outcome have considerably changed during the past two decades [70]. This syndrome could now be defined as persistent diarrhea despite prolonged bowel rest requiring long term-total parenteral nutrition (TPN) in children when no effective treatment is available [71, 72]. According to that definition, EN in this circumstance is indicated and often effective [73]. In fact, the reduction of digestive secretions, villous atrophy, and acquired brush border disaccharidase deficiency lead to malabsorption and malnutrition . Most of the time, a short course of PN followed by protracted CEN provides control of the disease within 6–8 weeks [74, 75]. In a prospective study of CEN versus PN, Orenstein showed that the resolution of diarrhea was faster in the enterally fed group [75]. The use of CEN in children and mainly infants with protracted diarrhea presenting also severe malnutrition may prove difficult. If the response to CEN is not good enough to provide rapidly adequate caloric supplies with resolution of diarrhea, PN should be started. Such decisions must be taken by experienced teams in specialized and well-staffed units. Severely malnourished infants with some types of particularly severe celiac disease , intolerance to cow milk proteins, protein hydrolysates, or with specific malabsorption syndromes, such as Anderson’s disease may also benefit from CEN [76].
Short Bowel Syndrome
SBS is the leading cause of intestinal failure in newborns as well as infants and young children, and it is most commonly the result of an extensive intestinal resection during the neonatal period. EN is often used in these circumstances, although several controversies over the ideal nutritional treatment of children with SBS remain [77]. Of note, intestinal failure may also lead to liver disease [78] (see also Chap. 43 on “Short Bowel Syndrome”).
Congenital and Newborn Intestinal Disorders and Pseudo-obstruction
In neonatal abdominal surgery for congenital or acquired disease, CEN, usually combined with PN, offers prolonged nutritional support which has transformed the prognosis in many conditions and is particularly important in the following situations: (1) reduction of the absorptive surface with enterocutaneous fistulae or extensive intestinal resection; (2) functional disorders of gut motility, such as malfunctions of duodenojejunal anastomosis, “plastic” peritonitis after repeated interventions, gastroschisis, and omphalocele.
Chronic intestinal pseudo-obstruction syndrome is a disabling disorder of enteral feeding characterized by repetitive episodes or continuous symptoms and signs of bowel obstruction, including radiographic documentation of dilated bowel with air–fluid levels, in the absence of fixed-lumen-occluding condition [79]. Pseudo-obstruction is classified as “congenital” if newborn infants present with symptoms persisting for the first 2 months of life. “Acquired” pseudo-obstruction applies when previously well patients present with feeding intolerance from pseudo-obstruction symptoms persisting longer than 6 months [79]. Most patients with intestinal pseudo-obstruction require decompression stomas, ileostomies, and colostomies to relieve the recurring obstruction symptoms. Long-term PN is required in a significant proportion of children with pseudo-obstruction [80–82], and even then, administration of at least small volumes of CEN is essential and encouraged for prevention of PN-associated liver disease [83] and maintenance of bowel mucosal integrity [84].
Crohn’s Disease
Enteral feeding has been used for many years, particularly in Europe, not only to improve nutritional status and growth but also to influence disease activity in patients with Crohn’s disease [85–94] . It was shown that CEN diet is as effective as high-dose corticosteroids in inducing remission in pediatric patients with Crohn’s disease and has the added benefit of improved growth and development without the steroid side effects [95, 96]. However, serial meta-analysis conducted by Griffiths et al. in 1995, 2001, and 2007 suggested that therapy with EN was inferior to corticosteroids for inducing clinical response in patients with active Crohn’s disease [97–99]. All three analyses largely included adult studies, and several pediatric studies showing striking efficacy were excluded owing to methodological weakness. A more recent meta-analysis that focused on 11 randomized controlled pediatric studies still reported similar efficacy for EN and corticosteroids for inducing remission in Crohn’s disease but also cited limited data [100]. The benefits of EN appear to be more favorable in children than adults including correction of impaired growth. Thus, EN is an underused therapy with the advantage of preserving growth while remission is achieved and therefore must be promoted [101]. There is no relevant data showing any significant difference in clinical outcome based on composition of the EN, that is, elemental, protein hydrolysates, or polymeric formula. Indeed, the beneficial effect in achieving remission appears not to be related to the mode of delivery or to the type of diet (no beneficial effect of elemental versus polymeric or semi-elemental) but rather to the reduced antigen load and mostly to changes in the intestinal microbiota [95]. Removal of antigenic material, alteration in intestinal micro flora, changes in gut hormone levels, and the presence of bioactive transforming growth factor-β (TGFβ-1) in casein-based formulas may all play a role in the clinical success of EN [102, 103]. Glutamine-enriched polymeric diet offered no advantage over a standard low-glutamine polymeric diet in the treatment of active Crohn’s disease [104]. EN may be also helpful in correction or maintenance of the nutritional state, especially during a relapse of Crohn’s disease [96] . EN is part of the preparation for surgical procedure and may be useful during recovery, especially after intestinal resections or enterostomies. In case of severe digestive involvement during Schönlein–Henoch purpura, CEN can be used as nutritional support in the absence of occlusion [105] More on the treatment of Crohn’s disease can be found in Chap. 28 .
Other Malabsorption Syndromes
Cystic Fibrosis
Malnutrition and impaired growth affects approximately 23 % of children with cystic fibrosis (CF) [106], and growth within the normal range of weight-for-length and BMI is associated with better pulmonary function and long-term survival. In 2008, the CF Foundation recommended that growth assessment using percent ideal body weight (% IBW) be discontinued, and instead rely on age-appropriate weight-for-length percentiles in children aged < 2 years, and BMI percentiles in children aged 2–20 years. The nutritional goals in children with CF are to maintain and support growth at weight-for-length or BMI at or above the 50th percentile [106]. Growth abnormalities in patients with CF have a multifactorial etiology including active CF-pulmonary or sinus disease, inadequate or ineffective pancreatic enzyme therapy, inadequate calorie intake, anorexia, presence of CF-related hepatobiliary disease, CF-related diabetes, infectious enteritis , small bowel bacterial overgrowth, and other comorbid intestinal conditions including, for example, celiac disease , inflammatory bowel disease and SBS [107]. Whereas these disorders require specific therapy, nutrition monitoring and intervention in patients with CF should always be implemented regardless of comorbid disease. Improved weight status in patients with CF often requires energy and protein intakes ranging from 110 to 200 % of normal needs in healthy people [108, 109]. However, even with an optimal approach to oral feeding, some patients respond poorly to nutritional counseling alone. Therefore, use of EN to supplement dietary intake is recommended to help restore and maintain nutritional status, especially in children aged 13 years and older who present with persistent growth deficits despite nutrition counseling [106]. The utilization of supplemental EN by patients with CF increased from 8.5 % in 2001 to 11.1 % in 2011 [110] thus indicating greater engagement of nutritional therapy. EN is generally performed during the night over 8–10 h with the initial goal of providing 30–50 % of the estimated requirements [107]. Patients with CF are then asked to eat and drink as much as possible during the daytime. Standard formulas containing intact protein, long-chain fat and calorie densities of 1.5–2.0 kcal/mL are generally well tolerated with appropriate dosing and administration of pancreatic enzymes [107, 111] Semi-elemental formulas may be tolerated better in patients with excessive anorexia, bloating, or nausea. The data are unclear whether formulas with medium-chain triglycerides are advantageous over regular formula in patients with CF [107].
Nasogastric tube (NGT) feeding is generally used as a first step. The tube is passed every night, 1–2 h after dinner, and removed in the early morning before physical therapy so that patients are not disturbed during the daytime for school attendance. In some children, NGT becomes increasingly uncomfortable because of nausea, vomiting, nasal discomfort as a result of nasal polyposis , or dislodgement during coughing in cases of pulmonary exacerbation. PEG is the preferred modality for administering home nocturnal EN when long-term EN is anticipated. Generally, PEGs are perceived well by patients with CF who rely on EN therapy for poor weight gain or weight loss. On the contrary, patients and families without PEGs were found to be apathetic towards their value, citing fear of interference with activities, embarrassment, pain, and discomfort [112]. Therefore, there is need for more accurate information about benefits and lifestyle in patients with PEGs. Good early childhood nutritional status is associated with better pulmonary function and long-term survival in patients with CF [113]. However, poor growth and advanced lung disease have been associated with poor outcomes regardless of adherence to EN [114]. There is also currently insufficient evidence to determine whether nutritional rehabilitation after onset of malnutrition improves pulmonary function in patients with advanced CF-related lung disease. Therefore, the importance of a proactive approach of growth monitoring and early nutritional intervention to optimize growth in children and adolescents with CF. It is important to assess for gastroesophageal reflux before starting EN. Patients should also be monitored for glucose intolerance during administration of EN, especially during illness, therapy with steroids, and if not gaining weight. Insulin therapy should be administered as needed in patients with glucose intolerance [107].
Chronic Liver Disease
Mechanisms leading to protein-energy malnutrition and requirement for EN in infants and children with chronic liver disease are multifactorial and dependent on the type of liver injury [115]. Malnutrition in cholestatic liver disorders is from reduced biliary secretion and intraluminal bile concentrations resulting in malabsorption of lipids and fat-soluble vitamins [116]. Protein and energy requirements are increased by different mechanisms including hypermetabolism [117], portosystemic shunting and ascites , futile metabolic pathways [118], and the energy demands from complications such as sepsis or variceal bleeding [119]. Liver disease from inborn errors of metabolism, for example, galactosemia, tyrosinemia, GSD, and Wilson’s disease requires specific dietary restrictions and EN feeding protocols to prevent hypoglycemia and other forms of metabolic decompensation. Patients with fulminant hepatic failure may be well nourished and nutritionally independent at clinical presentation but later require EN because of impaired mental status. Anorexia is common in children with chronic liver disease resulting from organomegaly, abdominal pressure effects of ascites, congested gastric mucosa, reduced motility from portal hypertension , central effects of unidentified toxins, dietary manipulations such as fluid restriction, or use of unpalatable feeds. Several factors contribute to long-chain polyunsaturated fatty acids (PUFAs) deficiency including low PUFAs intake, malabsorption, and disturbed metabolism of long-chain PUFAs. Finally, the interaction of growth hormone with insulin-like growth factor 1(IGF-1) and its binding proteins constitute an important mechanism linking nutrition and growth .
The most common cause of cirrhosis in children is biliary atresia , and the only definitive therapy is liver transplantation [120]. A total of 60–80 % of affected children are moderately to severely malnourished prior to transplantation [116], and poor nutritional status prior to transplantation is associated with prolonged hospital stay, increased risk for death at high cost of medical care [119, 121, 122]. Supplemental EN or PN to improve nutritional status is the one intervention known to improve pre- and posttransplant growth and clinical outcomes in children with end-stage liver disease [123–127]
Anorexia, inadequate calorie intake, and failure to thrive are prevalent in children with chronic liver disease [116]; therefore, EN is recommended to supplement per oral food intake [119]. NGT feeding may be safely used without increased risk for bleeding in patients with portal hypertension varices [128, 129]. PEGs should be avoided in children with chronic liver disease and portal hypertension because of likely portal-hypertensive gastropathy with increased bleeding risk from gastric varices [119]. MCTs are nutritionally advantageous in patients with cholestasis because of no bile requirement for digestion and rapidly absorbed into the portal circulation. MCTs may be administered separately as supplements; however, the selected EN should not contain more than 80 % of fat as MCT because of risk for inducing essential fatty acid deficiency [130] . Children with weight-for-length z scores < − 3 fall into the category of severe malnutrition and > 9-fold risk for mortality [131] therefore should be prioritized for nutritional intervention. Patients with chronic cholestatic liver disease have increased metabolism [117], futile metabolic pathways [118], and malabsorption; therefore, the goal for supplemental EN to enable a daily calorie intake of 140–200 % of estimated requirements [116]. Children with chronic cholestatic liver disease should continue receiving fat-soluble vitamins (A, D, E and K) regardless of amounts listed in EN [132]. Protein intake of infants and children should not be restricted except in cases of intractable encephalopathy [132], and, even in these cases, protein intake should remain within the adequate intake range of 1–2 g/kg/day [133] and accompanied by sufficient intake of nonprotein calories to prevent inappropriate utilization of protein for energy synthesis [134]. Children with cholestatic liver disease have increased requirements for branched-chain amino acids (BCAA) compared to healthy controls [135]. However, nutritional outcome studies using either BCAA-enriched or standard formulas reported growth benefits in all recipients of EN [123, 124]. Furthermore, a Cochrane review did not find BCAA-enriched formula protective against encephalopathy when compared to iso-nitrogenous non-BCAA enriched formulas [136] Therefore, there is currently insufficient evidence to recommend the use of BCAA-enriched formulas over standard non-BCAA enriched formula .
Chylothorax
Nutritional management of chylothorax involves adherence to EN or an oral diet enriched with medium-chain triglycerides and restricted in long-chain triglycerides and supplementation with essential fatty acids and fat-soluble vitamins [137]. Nutrition support during the preoperative period utilizes EN in infants who are hemodynamically stable and early use of PN if otherwise. The immediate postoperative period is characterized by fluid restrictions and hemodynamic instability and therefore requires active involvement of a dietician and reliance on PN to meet goal calories. Once hemodynamic stability is established, enteral feeding is introduced while following standardized protocols that include screening for swallowing dysfunction and management of GI symptoms, for example, reflux. Nutrition surveillance should be performed continually pre- and post discharge to guide further interventions including use of calorie-dense formula and home tube feeds [62].
Techniques of Delivering Enteral Nutrition
The route of EN administration should be individually tailored, depending on the underlying condition (Fig. 46.1). Enteral feeding is preferable to PN, and therefore it should be immediately implemented in children with functional GI but unable to feed per oral. The absolute contraindication to EN includes mechanical obstruction of the GI tract (unless indicated for decompression), active peritonitis, uncorrectable coagulopathy, or bowel ischemia [138, 139]. The intragastric route of administration is the most commonly used in children, since it is the more physiologic route which permits the action of salivary and gastric enzymes, the bactericidal action of gastric acid, and the better mixing with biliary and pancreatic juice. Therefore, the duodenal or jejunal route is used in very few circumstances in children. For intragastric EN, NGT, or GT may be used. NGT feeding is the best initial approach to EN, to evaluate the tolerance of EN before placing a permanent GT, and/or when a brief period of EN support is anticipated.
NGT are made of polyvinylchloride (PVC), polyurethane, or silicone. Modern feeding tubes are made of either silicone or polyurethane [140]. Polyurethane tubes come externally and internally impregnated with a water-activated lubricant to ease insertion through the nasopharynx and facilitate removal of introducer wires [141]. They are also more resistant to degradation and deterioration when compared to silicone tubes. Tube size (outer diameter) is described in French Gauge (Fr) units. The millimeter conversion can be derived by dividing each Fr by π (3.14). The length of insertion for a nasal- or oral-gastric feeding tube to be in a child is determined by using either the morphological markers of “nose–ear–mid-xiphoid–umbilicus” span or age-specific prediction equations. Length predictions based on just a ‘”nose–ear–xiphoid” span are likely to result in a placement that is too proximal [142]. At the time of NGT placement, aspirating fluid and measuring a pH ≤ 5 is the most reliable bedside test confirming gastric placement in children [143]. However, the usefulness of this test may be limited in patients being treated with gastric acid suppressants. Simple auscultation is not a reliable method for assessing position because injection of air into the tracheobronchial tree or pleural space can produce an indistinguishable sound. Therefore, an abdominal X-ray is the gold standard for establishing location of the NGT tip [143].
Duodenal or jejunal tube placement is more difficult; the patient should be placed in the right lateral position, and if necessary, after an intravenous injection of erythromycin. The position of the distal end of the tube is then checked by abdominal X-ray. Careful nasal fixation of the tube is used to avoid displacement; it is taped to the upper lip, the ipsilateral cheek, and the external ear. In some particular indications for EN, the feeding tube may also be introduced through the mouth, especially in patients with congenital or acquired nasal obstruction and premature infants on respiratory therapy with continuous positive air pressure (CPAP). The placement of NGT made of PVC is easier, but these tubes should be changed more frequently as they become more rigid. Silicone or polyurethane NGT may be used over 3-week periods or more. However, silicone and polyurethane are generally more flexible and easily displaced by vomiting. They are preferentially used for transpyloric and long-term EN. Individualized goals for growth, nutrition, and when to discontinue EN should be established prior to insertion of a feeding tube. Use of EN may only be temporary in acutely ill but previously healthy children, or as a long-term source of supplemental nutrition in children with chronic inadequate per oral food intake, and the sole source of nutrition in children with severe disability in per oral feeding.
When a child’s duration on EN is longer than 30 days and feeds well tolerated yet low anticipation for timely acquisition of adequate per oral feeding skills, transition to a feeding gastrostomy should be considered [138, 140]. Percutaneous approach to placement of gastrostomy has revolutionized the placement of enteric feeding tubes in children. The standard approach involves use of a gastroscope for gastric transillumination and stoma site identification. This is followed by percutaneous introduction of a guide wire that gets grasped using the gastroscope and then orally withdrawn. Thereafter, the gastrostomy catheter is attached to the oral end of the guide wire, and then pulled in an antegrade manner through the oropharynx, esophagus, and stomach, and then across the gastric and abdominal walls [144]. It may be placed using GI endoscopy, surgical laparoscopy , or by interventional radiology [140]. Following placement, postoperative care mostly involves pain management, and most providers permit the use of GT within 12–24 h. The initial PEG tube is changed after 2–3 months, by which time a good tract has formed. Button-replacement gastrostomy devices provide patients a cosmetic advantage in case of long-term EN.
Percutaneous placement of gastrostomy or jejunostomy is contraindicated in patients with previous abdominal surgery, abnormal abdominal anatomy , or severe deformities of the chest and spine which modify the position of the stomach and other intra-abdominal viscera. In such cases, a surgical GT should be placed. The implantation of a jejunal feeding tube, via PEG, is a possible method for the treatment of inadequate oral feeding in patients who are affected by gastroesophageal reflux (GER) and is thus an alternative to fundoplication and drugs [144, 145]. However, gastrojejunal feeding tubes are also prone to technical complications requiring replacement because of clogging and recoil of the jejunal catheter back into the stomach [145]. EN may be delivered as boluses or prolonged continuous feeding using a feeding pump, syringe, or controlled delivery by gravity. Pumps recommended for pediatric use have to provide clear flow rate display and alarms. Miniaturized and battery-powered pumps are specially designed for home and ambulatory EN.
Nutrients
Nitrogen
The absorption of amino acids is more rapid and efficient when given in the form of short peptides than free amino acids [146, 147]. Therefore, in order to maximize nitrogen assimilation in patients with marked impairment of gut absorptive capacity, the ideal EN should consist of di- and tripeptides and free amino acids [147]. In addition, the quality, in term of digestion and intestinal absorption of protein hydrolysates, depends on the type of hydrolysate, for example, lactalbumin is superior to casein [148]. However, in patients with normal GI function, EN with formulas consisting of protein hydrolysates offers no nutritional or absorptive advantage over EN based on formula consisting of free amino acids or intact protein [149]. Thus, the initial formula in patients with normal GI function should be based on intact protein or polypeptides, with a lower osmolality, rather than on a mixture of free amino acids and short polypeptides.
Carbohydrates
Disaccharidase enzymatic activities are depressed in disease involving the small intestine mucosa. Lactase appears to be the most sensitive to injury and the last of the disaccharidases to recover. In addition, certain drugs such as neomycin or colchicine depress the intestinal disaccharidases. Thus, it is important to avoid dietary sources of lactose. Other disaccharides should also be omitted from the solution used for initial feeding as their corresponding brush border enzymatic activities are reduced. The carbohydrate source allowed during the EN in patients with normal GI function can be lactose. However, patients with impaired GI function should be fed an EN containing lacto-free glucose polymer as the carbohydrate source.
Lipids
The main dietary lipids are triglycerides structurally made up of three fatty acids linked to glycerol molecule. The fatty acids contain between 2 and 24 carbon atoms (C: 2 and C: 24). Classification of the triglycerides is based on length of the fatty acid chain, that is, short-chain triglycerides (SCT) contain fatty acids that range in length from 2 to 4 carbons (C: 2 to C: 4); the MCT contain fatty acid chains ranging from 6 to 12 carbons (C: 6 to C: 12) and; LCT have > 12 carbons. In contrast to the LCTs, dietary MCTs are hydrophilic and do not require bile salts or micelle formation, and their free fatty acids are directly absorbed into the portal systems without requirement for re-esterification into chylomicrons [14, 150]. MCTs are hydrolyzed faster than LCTs in the small intestine by pancreatic lipase; they are converted almost exclusively into free fatty acids and glycerol and reach directly the portal circulation and the liver. Nevertheless, in case of pancreatic insufficiency, MCTs may also be absorbed intact. The excessive use of MCTs-containing diet can lead to osmotic diarrhea as a result of their rapid hydrolysis. Dicarboxylic aciduria has been described in infants supplemented with MCTs-rich formulas without any proof of deleterious effect [151]. The provision of essential fatty acids (EFA) must be considered since MCTs contain no EFA. Furthermore, MCTs decrease the LCTs absorption; thus, supplementation with linoleic acid must be done. However, its addition to a formula based on MCTs may be insufficient to prevent EFA deficiency, thus making necessary to provide EFA parenterally. Nevertheless, most of formulas containing MCTs include also up to 50 % of lipid as LCTs. LCTs in excess in the intestinal lumen, especially if they are hydroxylated by bacteria, reverse the rate of water and electrolyte absorption and increase malabsorption . In those conditions, the addition of cholestyramine an EFA supplement may be appropriate. Finally, a lipid intake of 3–4 g/kg/day may be achieved, depending on absorption capacity and digestive tolerance.
Other Components
Recommendations concerning energy, water, and electrolytes supplies in premature and full-term infants are provided in the chapter on PN in the premature infant. In older children, the recommended energy intake is based on recommended daily intake (RDI) values or may be estimated using World Health Organization (WHO) weight, age, and gender prediction equations or directly measured using an indirect calorimeter [133]. The minimum daily fluid requirements with some exceptions may be estimated using the “Holliday–Segar” calculation, which is an extrapolation based on daily calorie expenditure. For weights ranging from 0 to 10 kg, the estimation is 100 mL/kg/d; from 10 to 20 kg, 1000 mL plus 50 mL/kg for each kilogram of body weight more than 10; and over 20 kg, the estimation is 1500 mL plus 20 mL/kg for each kilogram more than 20 [152].
Choice of a Formula
Enteral feeding formulas are divided into several families. The choice of a formula is made according to numerous parameters like protein–calorie needs, digestive function, protein sensitivity, motility status, tolerance to fluid intake, all obviously dependent on the age and on the underlying disease (Fig. 46.3). In preterm, newborns, and young infants with normal intestinal function, human milk or standard infants formulas supplemented with long-chain polyunsaturated fatty acids (LCPUFA) may be used. Formulas for preterm infants are unique in being more calorically dense (72–90 kcal/100 mL) with increased protein (1.8–2.3 g/100 mL), calcium (70–108 mg/100 mL), and phosphorous [153]. The preterm formulas are continued after hospital discharge in preterm or small for gestation-age infants with discharge growth parameters are below appropriate post-conception growth, and continued until achievement of catch-up growth [153]. Breast milk and standard infant formulas have a calorie density of 0.67 kcal/mL with lactose as the carbohydrate source and fat source compromised of LCTs and LCPUFA (DHA and EPA). Commercial polymeric formulas are available for older children (age > 1 year), and blenderized diets can be prepared using food from the kitchen may be used in the nonstressed patients, with normal gut function. The calorie density of formulas used in older children (age > 1 year) ranges from 1 kcal/mL (standard) to 1.5–2.0 kcal/mL (calorically dense).
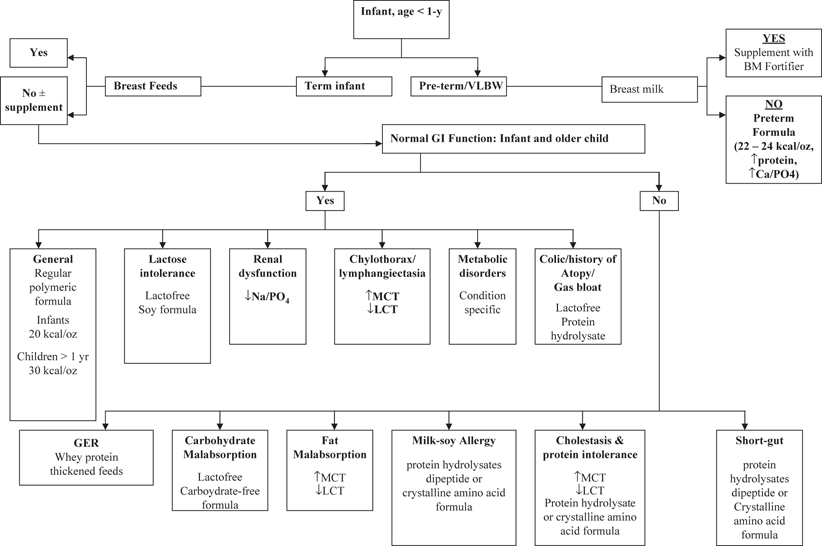 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







