Fig. 26.1.
Example of pre-written postoperative medical orders for postoperative day 2 after esophagectomy.
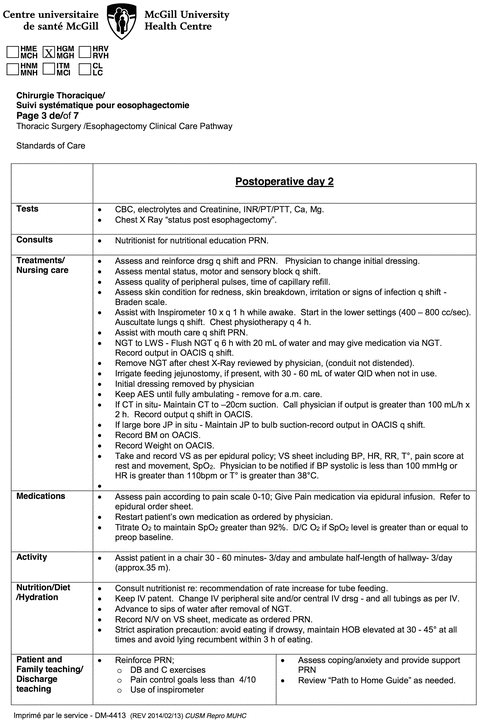
Fig. 26.2.
Example of pre-written postoperative nursing care map for postoperative day 2 after esophagectomy.
Elements of the McGill University Esophagectomy Enhanced Recovery Program
Our initial enhanced recovery pathway for esophagectomy was implemented for all esophagectomy patients in June 2010 with an initial target of discharge on day 7. This pathway has evolved and undergone incremental changes over the years to each of its elements. The current pathway is described in Table 26.1 and has a target discharge of postoperative day 6.
Table 26.1.
Summary of the elements of the enhanced recovery program for esophagectomy.
Pre- and intraoperative | |
Nutritional management | • Routine nutritionist consultation at time of diagnosis and during neoadjuvant therapy • Fast-track neoadjuvant chemotherapy to enable greater oral intake [10] |
Patient education | • Education booklet provided • Web-based interactive program provided • Pathway is reviewed with patient at preoperative visit |
Analgesia | • Routine dual-thoracic epidural catheter insertion to cover abdominal land thoracic fields [12] |
Fluid management | • Avoid fluid overload, balanced fluid administration—aim for (4–6 ml/kg/h) |
Minimally invasive approach | • Selected for high-grade dysplasia or early clinical T1–T2 N0-stage cancer |
Postoperative | |
Intensive care unit | • Extubation in the operating room and observation in post-anesthesia care unit for 6 h • Avoidance of routine intensive care unit admission |
Thoracic drainage | • Avoidance of rigid chest tubes and pleural drainage systems • Use of one large-capacity (400 ml), large-bore (19Fr) soft closed suction drain • Remove thoracic drain when full diet tolerated and drainage < 450 ml/24 h |
Conduit decompression | • Nasogastric tube removed on postoperative day 2 if no conduit over-distension on X-ray |
Urinary catheter | • Removed on postoperative day 1 |
Oral intake | • No routine surgical jejunostomy • Once nasogastric tube removed (postoperative day 2): – Water on postoperative day 2 – Clear fluid diet on postoperative day 3 – Solid post-esophagectomy diet on postoperative day 5 |
Radiology | • Daily portable upright chest X-ray until removal of chest tube • No routine contrast esophagram prior to solid intake [18] |
Mobilization | • Every postoperative day: incentive spirometry every hour when awake • Day 1: Sit in chair for 30 min 2 times, ambulate ½ length of hallway 2 times • Day 2: Sit in chair for 30 min 3 times, ambulate ½ length of hallway 3 times • Day 3: Sit in chair for 60 min 3 times, ambulate full length of hallway 4 times • Day 4–5: Sit in chair for 60 min for all meals (3 times), ambulate full length of hallway 4 times • Day 6: Discharge |
Preoperative Nutritional Management
Poor preoperative nutritional status is a concern in this patient population, as esophageal cancer frequently presents with dysphagia and significant weight loss. All new diagnoses of esophageal cancer are seen by a dedicated upper GI cancer nutritionist on the first clinical visit to discuss strategies to maintain appropriate nutritional intake using protein-rich drinks. Patients with locally advanced adenocarcinoma (T3 or N1) and appropriate performance status are referred for neoadjuvant chemotherapy based on a multicenter phase II trial [9]. We have found that symptomatic patients generally respond rapidly to neoadjuvant treatment and dysphagia lessens frequently within 1 week of starting treatment, enabling better oral intake [10].
Patient Education
Core to the success of an enhanced recovery pathway is the education of the patient and the family starting in the preoperative setting. Informing the patient on the postoperative process and managing patient expectations increases compliance to the pathway. At a preoperative visit, the expected postoperative course is reviewed with the patient using an easy to follow pictorial depiction of the pathway course covering activities, nutrition, drain management and pain control (Fig. 26.3 and http://www.muhcpatienteducation.ca/surgery-guides/surgery-patient-guides.html?sectionID=31). This educational process is further enhanced by the presence of a comprehensive and interactive web-based care module that covers the entire patient trajectory for this complex disease (http://www.muhcpatienteducation.ca/cancer-guides/cedars-cancer-guides/esophageal-cancer.html?sectionID=25&guideID=24).
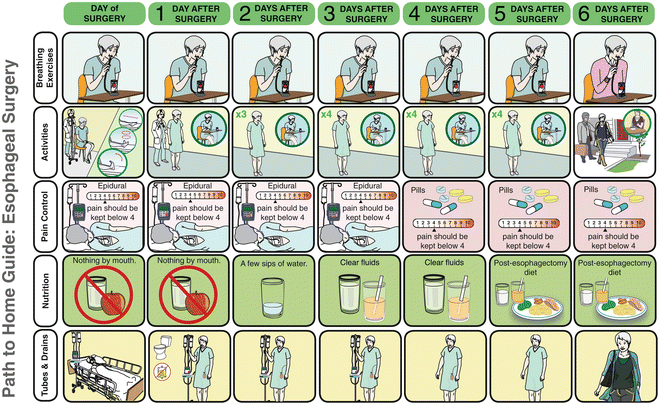

Fig. 26.3.
Patient educational material with pictorial representation of expected esophagectomy pathway course. The full booklet is available at http://www.muhcpatienteducation.ca/surgery-guides/surgery-patient-guides.html?sectionID=31 (courtesy of McGill University Health Centre Patient Education Office, Montreal, QC, Canada.).
Minimally Invasive Approach
We use a selected approach to surgery that is dependent on both patient disease and patient performance status. For patients with a clinical T1 or T2 N0 disease or for patients with high grade dysplasia, we offer a minimally invasive approach which is advantageous in decreasing the surgical stress response and lessening postoperative pain. Although minimally invasive esophagectomy does not appear to reduce mortality or pulmonary complications, there is a trend towards decreased intraoperative blood loss and decreased length of stay [11]. For more advanced disease requiring a 2-fields approach, it is possible to respect oncologic principles by doing the intra-abdominal mobilization by laparoscopy but to create the conduit, resect the thoracic disease, and perform the anastomosis using an open approach.
Epidural Anesthesia
Effective pain control is the cornerstone of expedited recovery. It reduces the risk of pneumonia, promotes early mobilization and decreases dependence on narcotics. Since the Ivor Lewis and 3-hole esophagectomies involve multiple dermatomes, optimal pain control targets both thoracic and abdominal regions. We use a dual-epidural catheters technique (thoracic and abdominal) that is associated with improved analgesia, decreased rate of major postoperative complications and decreased length of stay, without increasing catheter-related adverse effects compared with single-catheter use [12].
Avoiding Intraoperative Fluid Overload
Intraoperative management that is rooted in judicious intraoperative fluid administration results in decreased morbidity and mortality. Transfusion of blood products has been associated with decreased long-term survival in esophagectomy patients, and this correlation is observed for other cancer surgeries as well [13]. Intraoperative fluid restriction facilitates extubation in the OR suite and also decreases pulmonary complications especially in the setting of thoracic surgery [14].
Avoidance of Intensive Care Unit
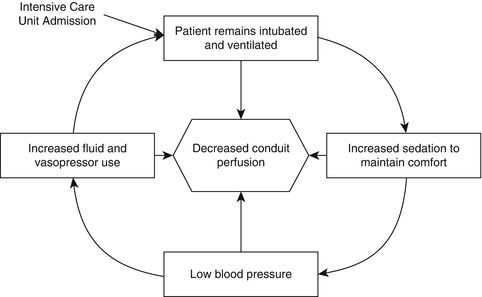
In traditional care, patients are often transferred from the operating room to an intensive care unit where they remain intubated and ventilated for up to 2 days. Remaining intubated in the intensive care unit restricts recovery as patients are unable to communicate and mobilize on their own. Moreover, keeping patients ventilated often induces discomfort that leads to a vicious cycle of increasing sedation, decreased blood pressure, and eventual compensation with over-resuscitation or vasopressor use which may stress blood flow to the esophageal conduit (Fig. 26.4).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






