(1)
Department of Visceral Surgery, University Hospital of Lausanne (CHUV), Lausanne, Switzerland
Keywords
Hepato-pancreato-biliary surgery and enhanced recovery pathwaysEnhanced recovery pathways and hepato-pancreato-biliary surgeryPancreatic surgery and enhanced recovery programsBiliary surgery and enhanced recovery programsIn the last two decades enhanced recovery pathways (ERP) have been successfully implemented in various fields of surgery, notably in colorectal surgery where numerous meta-analyses have shown lower complication rates associated with reduced postoperative stay and diminished hospital costs [1, 2]. Following these encouraging results, ERP have been progressively implemented to hepato-pancreato-biliary (HPB) surgery, which is traditionally considered as high-risk surgery.
Review of the Current Literature
Although pancreatic surgery has become safer in high-volume specialized centers with a significant reduction of perioperative mortality to 5 %, reported morbidity rates still remain considerably high, ranging from 40 to 60 % with a postoperative length of stay ranging from 14 to 20 days after pancreaticoduodenectomy [3]. Delayed recovery is mainly due to pancreatic fistula and delayed gastric emptying. A recently published systematic review in pancreatic surgery found that ERPs were associated with both a significant decrease in length of stay of 2–6 days and a reduction in complications without increased mortality or readmission rate [3]. However, the occurrence of pancreatic fistula or delayed gastric emptying did not differ [3]. On the other hand, the pathways were heterogeneous and important data like time to functional recovery and compliance to the ERP elements were scarcely described. Therefore, future prospective studies based on the published guidelines for perioperative care for pancreaticoduodenectomy by the ERAS® Society are required in order to assess the proper impact of ERP on functional postoperative recovery [4].
ERP have also been recently implemented in liver surgery, which is associated with about 40 % morbidity, with specific complications like hemorrhage, biliary leakage, intra-abdominal abscess and liver failure. In a meta-analysis [5], hospital length of stay was reduced and functional recovery was accelerated without compromising morbidity or mortality rates, and readmission rates were similar. In a recent randomized controlled trial on ERP for open liver resection [6], the median time to be medically fit for discharge was reduced with the ERP from 6 to 3 days, as was overall length of stay (7 vs. 4 days). The medical complications were significantly reduced, while surgical complications were similar. The readmission rates and mortality remained unchanged but health related quality of life in the first month after surgery was significantly better in the ERP group. However, there is until today no standardized protocol, and the guidelines on liver resection perioperative care from the ERAS Society are pending.
Specific Items in ERP for Pancreatic and Liver Surgery
In the patient population undergoing HPB surgery, preoperative nutrition is a significant concern. A nutrition screening assessing the Nutritional Risk Score (NRS) [7] is routinely performed, and malnourished patients with a NRS ≥3 are referred to specific dietician consultation. As the majority of advanced HPB surgery is still performed by laparotomy, preoperative immunonutrition (Oral Impact®, Nestlé) is given for 7 days before the surgery, as this intervention may reduce the infectious complications rate [8].
In order to implement ERP in liver resection, there are two major elements requiring some adaptation from those applied in colorectal surgery: fluid management and prophylactic drainage. In liver surgery, a relative hypovolemia with a low central venous pressure less than 5 cm H2O is maintained before and during liver resection, in order to minimize the amount of intra-operative blood loss. The blood pressure is controlled by vasopressors and blood transfusion when required. The central venous pressure can be lowered by the use of intravenous nitroglycerin during the liver resection. Once the resection is completed, euvolemia, as assessed by the central venous pressure, is restored by balanced crystalloid and colloid infusion, and hypoalbuminemia less than 20 g/L is compensated by intravenous albumin (20 %). For open liver surgery, a high thoracic epidural (T5–8) is placed and continued in the post-resection phase. Although not used in our center, a prophylactic drain placed close to the hepatic resection surface is still widely used, with the idea to prevent intra-abdominal collection, detect postoperative bleeding and bile leakage, as well as to drain ascites. However, a Cochrane Review did not show any statistically significant difference in terms of occurrence of postoperative infection or biloma or detection of bile leak and hemorrhage between the drain and no drain groups after elective liver resection [9]. Moreover, in a separate meta-analysis, there was a trend toward an increased rate of infected collections for drained patients [10]. There is currently no evidence to support the routine use of prophylactic drainage after liver resection. However, the use of abdominal drainage in order to prevent the accumulation of ascites, which can lead to ascitic leakage and wound dehiscence, remains debated in the current literature [11, 12]. Further trials are needed to assess its use in the specific group of cirrhotic patients.
In order to apply enhanced recovery principles to pancreatic surgery, and especially to pancreaticoduodenectomy, there are two main issues, which differ widely from colorectal ERP pathways: prophylactic drainage and postoperative nutrition. Following pancreatic resection, the use of prophylactic drains, placed in relation to both the biliary and pancreatic anastomoses, is still considered mandatory by many experts. Up to now there is only one randomized trial comparing prophylactic drainage vs. no drainage after pancreatectomy for pancreatic cancer [13]. This study found no significant difference in the mortality or the overall rate of complications whether intraperitoneal drains were present or not. Furthermore, drained patients were significantly more likely to develop an intra-abdominal collection or fistula (pancreatic and enterocutaneous). However, these data arise from a highly selected population of patients with pancreatic tumors treated in a specialized and experienced cancer center. In a meta-analysis comparing early vs. late drain removal, the incidence of pancreatic fistula was significantly lower in the early-removal group for patients at low risk of pancreatic fistula (amylase value in drains ≤5000 U/L at postoperative day 3) [14]. A recently published randomized multicenter trial comparing pancreaticoduodenectomy with or without routine drainage was interrupted by the data safety monitoring board because of increased mortality in the patients without drainage [15]. However, in this study all patients were randomized, irrespective of the pancreas consistency or the pancreatic duct size, and further trials specifically assessing the use of drainage in patients with higher risk of pancreatic fistula are warranted. Another intervention frequently used to prevent pancreatic fistula is somatostatin analogue, which reduces splanchnic blood flow and pancreatic secretion. In the current literature, somatostatin and its analogues do not reduce the rate of clinically significant fistula or overall morbidity and mortality [16] and are not systematically recommended [4]. Recently, new somatostatin analogues with longer half-life and broader binding profile such as pasireotide have been developed, and their use was found to be associated with a reduction of the rate of clinically significant pancreatic fistula following pancreatic resection in a randomized trial of 300 patients [17]. Further trials, with subgroup analyses specifically assessing the pancreatic texture and duct size, are necessary to evaluate the role of systematic somatostatin analogues in the prevention of postoperative pancreatic fistula.
Postoperative nutrition after a pancreatic resection is a key issue. A naso-gastric tube is routinely placed during surgery in order to evacuate air. However, there is high-level evidence that prophylactic nasogastric decompression increases the risk of pulmonary atelectasis and pneumonia and alters return of bowel function [18]. Therefore, the prophylactic use of nasogastric tube postoperatively should be avoided. In the postoperative period, a recent multicenter randomized controlled trial in patients undergoing major upper gastrointestinal and HPB surgery, including 82 pancreaticoduodenectomy, concluded that allowing early diet was safe for these patients and that enteral tube feeding did not confer benefit [19]. Therefore, patients should be allowed to gradually increase oral food intake over 3–4 days according to their tolerance. The use of enteral or parenteral nutritional should be reserved for patients developing major complications, with parenteral nutrition indicated only in those patients who cannot tolerate enteral nutrition [4]. A frequent complication after pancreaticoduodenectomy is delayed gastric emptying which can occur in up to a quarter of the patients. If prolonged delayed gastric emptying occurs, it may be necessary to insert a naso-jejunal feeding tube. In this case, supplemental nutrition should be established within ten postoperative days in order to resume a regular diet sooner [20].
Practical Implementation of ERP in Hepato-pancreato-biliary Surgery
Following successful implementation of ERP in elective and emergency colorectal surgery in our Department starting 2011 [21, 22], ERP was introduced in October 2012 for elective pancreas surgery and in June 2013 for elective liver surgery. Separate pathways were implemented for pancreaticoduodenectomy and spleno-pancreatectomy, as the latter, which does not include any digestive anastomosis, is less prone to delayed gastric emptying. For liver surgery, all different types of resection up to four segments were included in a single pathway. Based on the previously introduced ERP team for colorectal surgery, a similar group was organized (Fig. 25.1). Under the direction of the chair of the Department for Visceral Surgery, the three pillars of the ERP team were the surgeons, anesthesiologists, and nurses. For pancreas and liver, the surgeons in charge of the respective units were designed as leaders of the team and supported by two to three designated surgeons. The anesthesia leader was the same as for the colorectal ERP and also benefited from the support of other anesthetists. On the nurses’ part, a dedicated ERP nurse was involved in each of these pathways. The administrative direction was involved from the beginning and played a substantial role in obtaining the required resources. In addition, nutritionists, physiotherapists, and stoma-therapists were also involved in regular ERP team meetings in order to monitor and improve our protocols, which are detailed on Table 25.1. Specific documentation including patient education booklets and logbooks where the patients record their own progress, anesthesia protocols, standardized care maps and medical orders, were established.
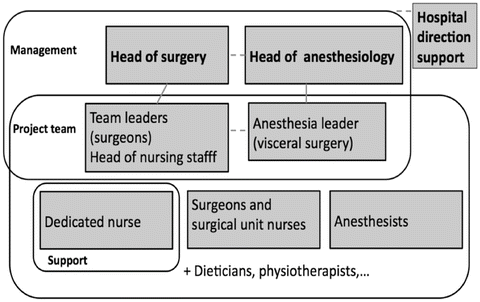

Fig. 25.1.
Organization chart of enhanced recovery team.
Table 25.1.
Perioperative care elements for hepato-pancreato-biliary surgery.
Liver resection | Duodenopancreatectomy | |
|---|---|---|
Preoperative counseling | Preadmission counseling (surgeon, dedicated nurse) + written information | |
Preoperative biliary drainage | – | Endoscopic biliary drainage if serum bilirubin higher than 250 μmol/L |
Preoperative smoking and alcohol consumption | Smoking and alcohol abstinence 1 month before surgery | |
Preoperative nutrition | Nutritional status assessment by the dedicated nurse (NRS score) and referral to dietician if at risk (NRS ≥3) | |
Oral bowel preparation | Avoidance of oral bowel preparation | |
Fasting | Clear fluids until 2 h, solids 6 h before surgery | |
Carbohydrate drinks | 800 ml on evening and 400 ml 2 h before surgery | |
Preanaesthetic medication | No long-acting sedative premedication | |
Antithrombotic prophylaxis | LMWH 12 h before surgery and continued for 4 weeks after surgery Intermittent pneumatic compression when in bed until POD 4 | |
Antimicrobial prophylaxis and skin preparation < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||



